UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
|
|
|
- Beatriz Alencastre Pedroso
- 6 Há anos
- Visualizações:
Transcrição
1 i UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCIELE OSMARINI LUNARDI VITRIFICAÇÃO E CULTIVO IN VITRO DE FOLÍCULOS SECUNDÁRIOS OVINOS: UMA ALTERNATIVA PARA A PRESERVAÇÃO DA FUNÇÃO REPRODUTIVA DE FÊMEAS FORTALEZA 2015
2 ii FRANCIELE OSMARINI LUNARDI VITRIFICAÇÃO E CULTIVO IN VITRO DE FOLÍCULOS SECUNDÁRIOS OVINOS: UMA ALTERNATIVA PARA A PRESERVAÇÃO DA FUNÇÃO REPRODUTIVA DE FÊMEAS Tese apresentada ao Ciências Veterinárias Universidade Estadual para a obtenção do Veterinárias. Programa de Pós-Graduação em da Faculdade de Veterinária da do Ceará, como requisito parcial grau de Doutor em Ciências Área de Concentração: Reprodução e Sanidade Animal. Linha de Pesquisa: Reprodução e Sanidade de Pequenos Ruminantes. Orientador: Profa. Dra. Ana Paula Ribeiro Rodrigues FORTALEZA 2015
3 iii
4 iv Dedico, A minha filha Bianca Lunardi Tronchoni, Que dobrou a alegria do meu viver. Ao meu marido Gustavo Tronchoni Que sempre esteve ao meu lado, principalmente nos momentos difíceis de execução desta tese. A minha mãe Que me deixou tranquila para excetuar e escrever a tese, ajudando-me a cuidar da minha filha durante a fase final doutorado.
5
6 v AGRADECIMENTOS À Universidade Estadual do Ceará (UECE) e ao Programa de Pós-Graduação em Ciências Veterinárias (PPGCV), aos professores e funcionários, por esses seis (seis) anos colaborando com a minha formação profissional. Ao Laboratório de Manipulação de Oócitos e Folículos Pré-Antrais (LAMOFOPA) da UECE, por dar-me todo o suporte para a realização dessa tese. À Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) pelo incentivo financeiro, através da bolsa de doutorado concedida. À minha orientadora, professora Dra. Ana Paula Ribeiro Rodrigues pela oportunidade de trabalho, por me orientar na execução desta tese e por me incentivar cada vez mais no exercício das minhas capacidades. Ao meu Co-orientador, professor Dr. José Ricardo de Figueiredo, pela oportunidade de ingressar em sua equipe, pela confiança depositada, por todos os ensinamentos e por acreditar no meu trabalho. Aos membros da banca Prof. Dr. Alexandre Rodrigues Silva, Profa. Dra. Cristiana Libardi Miranda Furtado, Prof. Dr. Dárcio Ítalo Alves Teixeira, Profa. Dra. Ana Beatriz Garcia Duarte e a Dra. Antônia Débora Sales pelas as correções desta tese contribuindo para torna-la ainda melhor. À Roberta Nogueira Chaves pelo exemplo como profissional, sempre muito responsável e ativa na busca pela qualidade e organização do funcionamento dos setores de sua responsabilidade. Também sou grata aos votos de confiança que ela me deu, incentivando-me a me organizar e procurar estar sempre à frente das demandas exigidas. À Laritza Ferreira de Lima por ter sido meu braço direito durante o período de experimento em que eu estava grávida da minha amada filha. Sem poder mexer em muitos reagentes, deveria ter ficado meses com a parte experimental parada. Porém meu anjo da guarda me enviou uma alma boa e competente para me auxiliar em diversas tarefas sem hesitar, muito obrigada. À Valdevane Rocha Araújo pela ajuda nas discussões de organização de protocolos, execuções de experimentos, bem como pelo companheirismo e amizade. À Anelise Maria Costa Vasconcelos Alves por me ensinar a realizar a técnica de histologia clássica de folículos isolados. Também sou grata a toda minha equipe do experimento I, pois todos foram essenciais para o êxito na publicação do primeiro artigo desta tese: Ivina Rocha Brito, Carlos Eduardo Azevedo Souza, Mariana Aragão Matos Donato, Christina Alves Peixoto e Andras Dinnyes. Ao professor Dr. Cláudio Cabral Campello pelo auxilio nas interpretações estatísticas dos resultados, bem como pela atenção e exemplo de conduta profissional.
7 vi À Ana Beatriz Graça Duarte pelas excelentes ideias, incentivo e auxílio na execução dos experimentos e correção dos artigos. Ao Francisco Léo Nascimento Aguiar que sempre soube me direcionar uma palavra sábia e motivadora, principalmente naqueles momentos em que mais precisamos onde nada parece dar certo. Também agradeço a sua contribuição como mente pensante me direcionando em questionamentos os quais na busca das respostas engrandeceram imensamente meus conhecimentos. À Livia Brunetti Apolloni que com sua bondade, delicadeza e serenidade trouxe alegria aos meados do meu doutorado, trazendo-me imensa ajuda na execução dos experimentos II e III (Fases II e III, respectivamente). À Jamily Bezerra Bruno que é uma excelente diplomata, intermediando diversas situações difíceis, tornando o ambiente mais leve e produtivo. Também agradeço as suas importantes ideias para a execução e escrita dos artigos dessa tese, bem como agradeço a dedicação e atenção demonstrada na correção desta tese. Ao Renato Félix da Silva pelo enorme auxílio na coloração das lâminas de histologia clássica de folículos isolados do artigo I. A toda equipe do LAMOFOPA que me auxiliou de alguma forma e as pessoas que fizeram meus dias mais felizes: Priscilla Campos, Nathalie Jiatsa, Rosane Oliveira, Rita Kelly, Marcela Pinheiro Paz, Lidiane Sales, Carolina Maside, Carlos Lobo, Lorena Andrade, Ívila Lorrine, Seu João, César Camelo, Julian Pontes, Luciana Mascena Silva, Anna Clara Accioly Ferreira, Johanna Leiva Revilla, Naiza Arcângela Ribeiro de Sá, Victor Macêdo Paes, Jesus de los Reyes Cadenas Moreno, Luis Albero Vieira, Hudson Henrique Vieira Correia, Kele Amaral Alves, Benner Geraldo Alves, Denise Damasceno Guerreiro, Deborah Magalhães Padilha, Viviane Saraiva, Rafael Rossetto, Antônia Debora Sales, Gerlane Modesto, Michelle Karen Brasil Serafin, Juliana Jales Celestino, Valesca Barreto Luz, Anderson Pinto, Cláudio Afonso Pinho Lopes, Rafael Rossetto de Sousa, Ana Kelen Felipe Lima; Ticiana Franco Pereira da Silva e Fabricio Sousa Martins. E um agradecimento especial ao Gustavo, meu querido marido, que teve muita paciência e soube suportar minha ausência em casa quando em muitas madrugadas precisei executar os meus experimentos e auxiliar em experimentos de colegas. Por cuidar da nossa filha, com muito carinho e dedicação, fazendo com que os momentos da minha ausência fossem momentos de alegria e convívio entre pai e filha e não em sentimento de perda por parte da minha filha. Muito obrigada meu amor! À Bianca a minha filha que me deu alegria, forças, motivação e me tornou uma pessoa mais organizada, produtiva e assertiva. Obrigada minha filha por lapidar o que há de melhor em mim, em
8 vii breve você saberá ler essas palavras de agradecimento, por enquanto te acolho com todo meu amor para manifestar que você é a pessoa mais importante do mundo para mim. E, por fim à minha mãe, Marli Osmarini, a pessoa fundamental para a finalização desta tese, que me acudiu no momento mais difícil da minha vida, auxiliando-me a cuidar da minha filha, durante o período final do doutorado.
9 viii "Um pouco de ciência nos afasta de Deus. Muito, nos aproxima." Louis Pasteur
10 ix RESUMO A criopreservação de folículos pré-antrais presentes no tecido ovariano (TO) tem sido utilizada com sucesso para restaurar a função reprodutiva após transplante, principalmente em mulheres submetidas a tratamentos gonadotóxicos. No entanto, o transplante do TO pode promover a reintrodução de células cancerosas. Desta forma, a utilização de folículos secundários (FS) (folículos pré-antrais em desenvolvimento) isolados do TO pode ser uma alternativa para evitar esse inconveniente. O principal objetivo desta tese foi identificar a melhor forma (isolada ou inclusa no TO) de vitrificação de FS ovinos para posterior cultivo in vitro e obtenção de oócitos maduros, visando à produção in vitro de embriões. Para isso, os FS foram avaliados in vitro após vitrificação em três diferentes fases: Fase I: Vitrificação de FS inclusos ou isolados do TO seguido de cultivo in vitro de curta duração (6 dias); Fase II: Vitrificação de FS inclusos ou isolado do TO seguido de cultivo in vitro de longa duração (18 dias) e maturação in vitro (MIV) e Fase III: Uso de diferentes meios (α-mem ou TCM199) para o cultivo in vitro de FS vitrificados inclusos ou isolados do TO, MIV e expressão gênica de CX37, CX43, BAX e BCL2. Os dados foram submetidos ao teste de normalidade de Bartlett s e/ou Shapiro-Wilke, as médias comparadas pelo teste de Tuckey e/ou Student s Newman Keuls, variáveis discretas ao Qui quadrado ou ao teste exato de Fisher, todos a uma probabilidade de erro menor que 5%. Na Fase I, observamos que FS vitrificados na forma isolada foram capazes de crescer, semelhantemente aos folículos frescos (P<0,05). Além disso, parâmetros como a morfologia, ultraestrutura, viabilidade, proliferação celular e percentual de formação de antro foram similares (P<0,05) entre os folículos vitrificados isolados ou inclusos no TO. Na Fase II, oócitos oriundos de folículos vitrificados na forma isolada retomaram a meiose a atingiram metáfase I (MI) semelhante aos folículos frescos ou não vitrificados (P>0,05). Na Fase III, observou-se que o meio α-mem foi fundamental para a retomada da meiose e metáfase II (MII). A expressão gênica para BAX e CX37 foi observada apenas nos folículos frescos cultivados em α-mem ou em TCM199. Já para o BCL2 a expressão foi significativamente superior nos folículos vitrificados no TO, cultivados em ambos os meios comparados aos demais folículos. Com relação à CX43, a expressão nos folículos frescos foi superior a dos vitrificados. Conclui-se que: 1) FS podem ser vitrificados com êxito antes ou depois do isolamento do TO, sem prejuízos para a morfologia e capacidade de formar antro após cultivo in vitro por 6 dias. No entanto, a ultraestrutura de folículos vitrificados é mais comprometida do que folículos frescos; 2) Oócitos provenientes de FS vitrificados na forma isolada e, cultivados in vitro por 18 dias se desenvolveram até o estágio de MI; 3) Ao final da MIV, o diâmetro oocitário não foi afetado pela vitrificação folicular ou meio utilizado; 4) Oócitos maturados in vitro provenientes de folículos vitrificados na
11 x forma isolada e cultivados in vitro em α-mem se desenvolveram até o estágio de MII e 5) A expressão gênica foi afetada pela vitrificação. Palavras - chave: Criopreservação. Tecido ovariano. Folículo isolado. Vitrificação. Maturação in vitro.
12 xi ABSTRACT The cryopreservation of preantral follicles enclosed in ovarian tissue (OT) has been applied successfully for the restoration of the ovarian function after transplantation mainly in women submitted to gonadotoxic treatments. However, the transplantation of the OT could cause reintroduction of cancerous cells. In this way, the utilization of secondary follicles (SF) (growing preantral follicles) isolated of the OT could be an alternative to avoid this bias. Therefore, the goal of this dissertation was to identify which is the best way (enclosed or denuded of OT) to vitrify ovine SF thereafter in vitro culture (IVC) and promotion of mature oocytes, aiming produce embryos in vitro. Thereby, the evaluation of SF from IVC after vitrification occurred in three different phases: Phase I: Vitrification of SF enclosed or denuded of OT, followed by short term IVC (6 days). Phase II: Vitrification of SF enclosed or denuded of OT, followed by long term IVC (18 days), and in vitro maturation (IVM); and Phase III: Utilization of different medium (α-mem or TCM199) for IVC of SF vitrified enclosed or isolated of OT, followed by IVM and gene expression of CX37, CX43, BAX and BCL2. The data applied Bartlett s and /or Shapiro Wilk. The media were compared using Turkey test and/or Student s Newman-Keuls, and discrete variable of the data used qui-square tests or Fisher s test. All probabilities where considered for errors minor than 5%. In Phase I, vitrified SF in an isolated form was capable to grow similarly to fresh follicles or non-vitrified (P < 0.05). Moreover, the end points morphology, ultrastructure, viability, cell proliferation and percentage of antrum formation were similar (P < 0.05) among vitrified follicles enclosed or denuded from OT. In Phase II, oocytes recovered from vitrified follicles in an isolated form had meiotic resumption reaching metaphase I (MI) similar of the fresh follicles or nonvitrified (P > 0.05). In Phase III, α-mem medium was prior to meiotic resumption and development until Metaphase II (MII) stage. The gene expression for BAX and CX37 were observed only in fresh follicles cultured in α-mem or TCM199. The BCL2 gene had higher (P < 0.05) expression in vitrified follicles enclosed in OT cultured in both medium when compared to other follicles. The CX43 gene had higher (P < 0.05) expression on vitrified follicles. As conclusions, 1) Vitrified SF can develop appropriately after or before isolation of OT, without any damage to morphology or capacity to form antrum after IVC for 6 days. However, the ultrastructure of vitrified follicles is more impaired than fresh follicles; 2) Oocytes from isolated SF vitrified and then IVC for 18 days developed reaching metaphase I stage; 3) At the end of IVM, no damage on oocyte diameters were observed neither by vitrification or by the base medium used; 4) IVM oocytes from vitrified follicles in an isolated form and IVC in α-mem developed to MII stage. 5) The gene expression was affected by vitrification. Keywords: Cryopreservation. Ovarian tissue. Isolated follicle. Vitrification. In vitro maturation.
13 xii LISTA DE FIGURAS Figura 1. Avaliação folicular por diferentes técnicas: (A) Micrografia de um folículo secundário na histologia clássica, (B) ultraestrutura de um folículo pré-antral, (C) folículos secundário viável marcado em verde pela calceína-am, (D) Folículo primordial corado pela técnica de AgNOR e (E-H) Oócitos marcados pelo Hoechst indicando as diferentes fases do processo de maturação nuclear oocitária. O: oócito, N: nucléolo, ZP: zona pelúcida, CG: células da granulosa, GV: vesícula germinativa, GVBD: quebra da vesícula germinativa, MI: metáfase I, MII: metáfase II Capítulo 2 Figure 1. Histological sections showing normal (a e) and degenerated (f) sheep follicles cultured for 6 days in Control (a, b) after Follicle-Vit (c, d) and Tissue-Vit (e, f). Normal follicles appear healthy with zona pellucida, follicular cells, theca cells, healthy basal membrane and spherical oocytes with uniform cytoplasm. Degenerate follicles display retracted oocytes, disorganized granulosa cells, and empty space in the oocyte cytoplasm. O oocyte, N oocyte nucleus, gc granulosa cells, ZP zona pellucida, TC thecal cells, A antrum Figure 2. Assessment of the viability of sheep secondary follicles cultured for 6 days using fluorescent probes. Tissue-Vit (a, b) and Follicle-Vit (c, d) classified as viable since cells were labeled with calcein-am (green fluorescence; b) and non-viable as cells were marked with ethidium homodimer-1 (red fluorescence; d), respectively. Note follicle containing 64 peripheral granulosa cells labeled with ethidium homodimer-1 (d). Figure 3. Ovine follicle diameter (μm) (mean ± SEM) (a), daily growth rate (μm/day) (mean ± SEM) (b) and the percentage of follicles with decreased diameter (c) before (day 0) and/or after 6 days of culture. Uppercase letters indicate statistically significant differences (P < 0.05) among treatments. Lower case letters indicate statistically significant differences (P < 0.05) between days of culture.. 65 Figure 4. Ovine oocyte diameters (μm) (mean ± SEM) of follicles from Control, Follicle-Vit and Tissue-Vit before or after 6 days of culture. Uppercase letters indicate statistically significant differences (P <0.05) among treatments. Lowercase letters indicate statistically significant differences (P < 0.05) between days of culture... 66
14 xiii Figure 5. Electron micrographs of ovine follicles. Different columns show follicles from Control (a, b), as well as from treatments Follicle-Vit (c, d) and Tissue-Vit (e, f), before (a, c and e) or after in vitro culture (b, d and f). In (a) (Control non-cultured), ultrastructural characteristics are well preserved, including regular and intact oocyte membrane and zona pellucida. Additionally, microvilli are visible in the zona pellucida. In (b) (Control cultured), microvilli are still visible, although in smaller numbers. Secretion vesicles shown in increased size and numbers compared to those from non-cultured control. In both controls, intact organelles were visible, especially mitochondria. After vitrification (c f), small irregularities in the zona pellucida and increased number of cytoplasmic vacuoles could be seen in follicles subjected to all treatments. In both vitrification and cultured treatments, there was a small decrease in the size and numbers of the microvilli between the oocyte and zona pellucida, and also low density of organelles and cytoplasmic material (d f). ZP zona pellucida, O oocyte, 67 gc granulosa cells Capítulo 3 Figure 1. Percentage of isolated morphologically normal sheep preantral follicles without vitrification (Control, n = 50) and after vitrification of isolated follicles (Follicle-Vit, n = 40) or ovarian tissue (Tissue-Vit, n = 64) at days 0, 6, 12, and 18 of in vitro culture. AB Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day. a,b Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group Figure 2. Normal sheep secondary follicle before (Day 0) in vitro culture (a) or cultured for 6 days (b), 12 days (c) or 18 days (d) in Follicle-Vit group. Note beginning of antrum formation at day 6 of in vitro culture. (e) Percentage of antrum formation of sheep preantral follicles without vitrification (Control, n = 39) and after vitrification of isolated follicles (Follicle-Vit, n = 39) or ovarian tissue (Tissue-Vit, n = 60) at days 6, 12, and 18 of in vitro culture. A,B Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day. a,b Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group. 87 Figure 3. Follicular diameter (µm) (mean ± SEM) of sheep preantral follicles without vitrification (Control, n = 21) and after vitrification of isolated follicles (Follicle-Vit, n = 38) or ovarian tissue (Tissue-Vit, n = 44) at days 0, 6, 12, and 18 of in vitro culture. A Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day. a,b,c Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group.. 88 Figure 4. (a) Daily growth rate (µm/day) (mean ± SEM) of sheep preantral follicles without vitrification (Control, n = 21) and after vitrification of isolated follicles (Follicle-Vit, n = 38) or ovarian tissue (Tissue-Vit, n = 44) after 18 days of in vitro culture and (b) Percentage of sheep preantral follicles with decreased diameter from Control (n = 18), Follicle-Vit (n = 1) e Tissue-Vit (n = 16) after 18 days of culture. A,B,C Different uppercase letters indicate statistically significant differences among groups (P<0.05). 89
15 xiv Figure 5. Viable oocyte in metaphase I after IVM from Follicle-Vit group after in vitro culture for 18 days. Note: Brightfield (a), calcein-am (b) and Hoechst (c). 89 Capítulo 4 Figure 1. Experimental design for vitrification and in vitro culture of sheep secondary follicles. SF: secondary follicles. IVC: in vitro culture. IVM: in vitro maturation. SF: 113 secondary follicles. IVC: in vitro culture. IVM: in vitro maturation.. Figure 2. Daily growth rate (µm/day) (mean ± SEM) of sheep preantral follicles without vitrification (Control α-mem, n = 32/47; and Control TCM199, n = 48/62) and after vitrification of isolated follicles (Follicle-Vit MEM, n = 57/68; and Follicle-Vit TCM199, n = 57/64) or ovarian tissue (Tissue-Vit α-mem, n = 58/82; and Tissue-Vit TCM199, n = 56/78) after 18 days of in vitro culture. A,B,C Different uppercase letters indicate statistically 114 significant differences among groups (P<0.05). Figure 3. Percentage of sheep preantral follicles with decreased diameter in groups without vitrification (Control α-mem, n = 15/47; and Control TCM199, n = 14/62) and after vitrification of isolated follicles (Follicle-Vit α-mem, n = 11/68; and Follicle-Vit TCM, n = 7/64) or ovarian tissue (Tissue-Vit α-mem, n = 24/82; and Tissue-Vit TCM199, n = 22/78) after 18 days of culture. A,B,C Different uppercase letters indicate statistically significant differences among groups (P<0.05) 114 Figure 4. Relative expression of connexin 37 mrna in ovine ovarian follicles. The expression of connexin 37 mrna was normalized to PPIA and the relative expression was presented as mean ± SD. Differences among groups were considered significant when P< Figure 5. Relative expression of connexin 43 mrna in ovine ovarian follicles. The expression of connexin 43 mrna was normalized to PPIA and the relative expression was presented as mean ± SD. Differences among groups were considered significant when P< Figure 6. Relative expression of BAX mrna in ovine ovarian follicles. The expression of BAX mrna was normalized to PPIA and the relative expression was presented as mean ± SD. Differences among groups were considered significant when P< Figure 7. Relative expression of BCl2 mrna in ovine ovarian follicles. The expression of BCL2 mrna was normalized to PPIA and the relative expression was presented as mean ± SD. Differences among groups were considered significant when P<
16 xv LISTA DE TABELAS Capítulo 1 Table 1. Folliculogenesis features in sheep and women Table 2. Advances in sheep ovarian tissue vitrification Table 3. Advances in women ovarian tissue vitrification Capítulo 2 Table 1. Number of fragments and follicles recovered from Control, Follicle-Vit and Tissue-Vit in each repetition Table 2. Percentages of morphologically normal, viable (calcein-am and ethidium homodimer-1) and antrum formation in sheep follicles in Control, Follicle-Vit and TissueVit before and/or after 6 days of culture. 68 Table 3. Average number of development NORs per nucleus of granulosa cells in sheep follicles in Control, Follicle-Vit and Tissue-Vit after 6 days of culture. 69 Capítulo 3 Table 1. Oocyte viability (%) and diameter (µm ± SD), recovery rate of oocytes cultured in vitro (%), and meiotic stages (%) of sheep oocytes from preantral follicles after long-term culture (18 days) in Control, Follicle-Vit and Tissue-Vit groups Table 2. 17β-estradiol (pmol/l) (mean ± SEM) and progesterone (nmol/l) concentration (mean ± SEM) in pooled media collected on the different days during long-term culture of preantral follicles in Control (n = 20), Follicle-Vit (n = 20) and Tissue-Vit (n = 20) groups. Capítulo 4 91
17 xvi Table 1. Oligonucleotide primers used for the real-time polymerase chain reaction analysis of ovine follicles Table 2. Percentage of isolated morphologically normal sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit αmem and Tissue-Vit TCM199) at days 0, 6, 12, and 18 of in vitro culture Table 3. Percentage of antrum formation of sheep preantral follicles without vitrification (Control MEM and Control TCM199) and after vitrification of isolated follicles (FollicleVit MEM and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit MEM and Tissue-Vit TCM199) at days 6, 12, and 18 of in vitro culture Table 4. Follicular diameter (µm) (mean ± SEM) of sheep preantral follicles without vitrification (Control MEM and Control TCM) and after vitrification of isolated follicles (Follicle-Vit MEM and Follicle-Vit TCM) or ovarian tissue (Tissue-Vit MEM and TissueVit TCM) at days 0, 6, 12, and 18 of in vitro culture Table 5. Oocyte recovery rate (%), viability (%) and meiotic stages (%) of sheep preantral follicles without vitrification (Control MEM and Control TCM) and after vitrification of isolated follicles (Follicle-Vit MEM and Follicle-Vit TCM) or ovarian tissue (Tissue-Vit MEM and Tissue-Vit TCM) Table 6. Oocyte diameter (µm ± SD) of sheep preantral follicles without vitrification (Control MEM and Control TCM) and after vitrification of isolated follicles (Follicle-Vit MEM and Follicle-Vit TCM) or ovarian tissue (Tissue-Vit MEM and Tissue-Vit TCM) after 18 days of in vitro culture and in vitro maturation.. 112
18 xvii LISTA DE ABREVIATURAS E SIGLAS 17β -HSD Hidroxisteróide desidrogenase A Antrum AA Ascorbic acid (ácido ascórbico) ACP/ CPAs Agente crioprotetor (cryoprotectant agents) AgNOR Argyrophilic proteins related to nucleolar organizer regions (proteínas argirofílicas relacionadas com regiões organizadoras de nucléolos) AI Anáfase I ANOVA Analysis of variance (Análise de variância) ART Assisted reproductive techniques (técnicas de reprodução assistida) BAX Bcl-2 associated X protein (proteína x associada ao Bcl-2) BCL2 B-cell lymphoma 2 (linfoma de células B 2) BCL-XL Bcl-2 related protein, long isoform (proteína relacionada ao Bcl-2 de longa isoforma) Bid BH3 interacting domain death agonist (agonista de morte com domínio de interação BH3) Bik Bcl-2 interacting killer (proteína exterminadora que interage com Bcl-2) BSA Bovine serum albumin (albumina sérica bovina) Calceína-AM Calceína acetoximetil camp Adenosina Monofosfato Cíclico (cyclic adenosine monophosphate) CAPES Coordenação de aperfeiçoamento de pessoal de nível superior cdna Complementary DNA CGP Células germinativas primordiais ckit Kit ligand receptor (receptor para kit ligand) CMIA Chemiluminescence microparticle immunoassay (imunoensaio de micropartículas por quimioluminescência) CNPq Conselho Nacional de Desenvolvimento Científico e Tecnológico COCs Cumulus-oocyte complexes (complexos cúmulos oócito) CT Delta-deltacycle threshold CV Coefficients of Variability (coeficientes de variabilidade) CV Conventional vitrification (Vitrificação convencional) CX 37, 43 Conexina 37, 43 (connexin 37, 43) DMSO Dimetilsulfóxido (dimethylsulfoxide) DNA Deoxyribonucleic acid (ácido desoxirribonucléico)
19 xviii dntp Deoxynucleotide DP Standard deviation (desvio padrão) DVC Direct cover vitrification (vitrificação convencional direta) EG Ethylene glycol (etilenoglicol) EGF Epidermal growth factor (fator de crescimento epidermal) FBS Fetal bovine serum (soro fetal bovino) FCS Fetal calf serum (soro fetal bovino) Fe(NO3)3 Nitrato férrico 9H2O Follicle-Vit / Follicle-Vitrification group (tratamento Vitrificação de folículos) Folículo-Vit FS Folículos secundários FSH Follicle stimulation hormone (hormônio folículo estimulante) G1 Gap 1 gc Granulosa cells GDP Guanosina difosfato GH Gowth hormone (hormônio do crescimento) GP130 Glycoprotein 130 (glicoproteína 130) GTP Guanosina trifosfato GVBD Germinal vesicle breakdown (quebra da vesícula germinativa ) HEPES 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid HSA Human serum albumin (albumina sérica humana) HTCM HEPES tissue culture medium IGF Insulin-like growth fator (fator de crescimento semelhante à insulina) IGF-I Insulin-like growth factor I (fator de crescimento semelhante à insulina I) IVF In vitro fertilization (fertilização in vitro) IVM In vitro maturation (maturação in vitro) KH2PO4 Fosfato de potássio monobásico KL Kit ligand LAMOFOPA Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (Laboratório de Manipulação de Oócitos e Folículos Pré-Antrais) LH Luteinizing hormone (hormônio luteinizante) LIF Leukemia inhibitory factor (fator inibidor de leucemia) LIF-R Receptor do fator inibidor de leucemia LN2 Liquid nitrogen (nitrogênio líquido)
20 xix MEM Meio essencial mínimo MI Metáfase I MII Metáfase II MOIFOPA Manipulação de oócitos inclusos em folículos ovarianos pré-antrais N Oocyte nucleus (núcleo do oócito) NORs Nucleolus organizer regions (regiões organizadoras de nucléolos) O Oocyte (oócito) OTC Ovarian tissue cryosystem P450arom P450 aromatase PAS periodic acid-schiff (ácido periódico-schiff) PEG Polyethylene glycol (polietilenoglicol) PFA Paraformaldehyde (paraformaldeído) PGC Primordial germ cells (células germinativas primordiais) PPIA Peptidylprolyl Isomerase A (cyclophilin A) PROH Propanodiol (propanediol) PVP Polyvinylpyrrolidone (polivinilpirrolidona) qpcr Quantitative polimerase chain reaction (reação em cadeia polimerase quantitativa) rbfsh Recombinant bovine follicle-stimulating hormone RNAm /mrna Àcido ribonucleico mensageiro (messenger ribonucleic acid) RNAr / rrna Ácido ribonucleico ribossômico (ribosomal ribonucleic acid) RT-qPCR Real time reverse transcription quantitative polimerase chain reaction (Transcrição reversa da reação em cadeia polimerase S Synthesis SAS Statistical Analysis System (Sistema de análise estatística) SCF Fator de células tronco SCID Severe combined immunodeficiency SEM Standard error of the mean (erro padrão da media) SNK test Student-Newman-Keuls test SSS Serum substitute supplement SUC Sucrose (sacarose) T4 Hormônios tetraiodotironina TC Thecal cells TCM199 Tissue Culture Medium 199 (meio de cultivo para tecido) TCM199B Meio de cultivo para tecido com bicarbonato
21 xx TCM199H Meio de cultivo para tecido com HEPES TGFb Transforming growth factor beta (fator de crescimento transformante beta) TI Telófase I Tissue-Vit / Tissue-Vitrification group (tratamento vitrificação de tecido) Tecido-Vit TO Tecido ovariano TUNEL Terminal deoxynucleotidil transferase-mediated deoxyuridine triphosphate biotin nick end-labeling VG Vesícula germinativa VS1 Vitrification solution 1 (solução de vitrificação 1) VS4 Vitrification solution 4 (solução de vitrificação 4) ZP Zona pellucida α-mem Alpha minimal essential medium (meio essencial mínimo alfa)
22 xxi SUMÁRIO 1 INTRODUÇÃO REVISÃO DE LITERATURA O ovário dos mamíferos: cenário da oogênese e da foliculogênese Atresia folicular A biotécnica de manipulação de oócitos inclusos em folículos ovarianos pré-antrais (MOIFOPA) Criopreservação de folículos ovarianos pré-antrais Avanços na vitrificação de folículos pré-antrais ovinos Cultivo in vitro de folículos pré-antrais Importância da composição do meio de cultivo Avaliação folicular após criopreservação e cultivo in vitro Avaliação morfológica Avaliação da funcionalidade JUSTIFICATIVA HIPÓTESES CIENTÍFICAS OBJETIVOS Objetivo geral Objetivos específicos CAPÍTULO Vitrificação de tecido ovariano de ovelha: um modelo para o estudo da preservação da fertilidade em mulheres CAPÍTULO Folículos ovinos secundários isolados vitrificados são capazes de crescer e de formar antro após curto período de cultura in vitro CAPÍTULO Folículos secundários ovinos vitrificados na forma isolada crescem e se desenvolvem in vitro melhor do que aqueles vitrificados em fragmentos de ovário CAPÍTULO Folículos ovinos secundários isolados e vitrificados são capazes de produzir oócitos em metáfase II após cultivo e maturação in vitro CONCLUSÕES PERSPECTIVAS REFERÊNCIAS BIBLIOGRÁFICAS
23 1 1 INTRODUÇÃO A conservação de material genético de animais vivos, especialmente, embriões, oriundos de fêmeas de alto valor tem sido uma preocupação permanente de vários pesquisadores (TUCKER et al., 2004; LAMBERTINI et al., 2015) em todo o mundo. No entanto, a perda de animais de maneira inesperada ou acidental é uma condição que deve ser considerada relevante, haja vista ser um evento comum nos rebanhos, notadamente naqueles de elite, frequentemente submetidos às exposições agropecuárias. Desta forma, uma alternativa para a conservação do material genético de fêmeas nessas circunstâncias é a criopreservação de oócitos inclusos em folículos pré-antrais obtidos a partir do córtex ovariano, dos quais, teoricamente, pode-se recuperar 90% do capital oocitário de um indivíduo. Porém, essa técnica ainda tem sido considerada experimental, cujos resultados revelam a necessidade de aprimoramento dos protocolos atualmente utilizados. Dentre os métodos de criopreservação de folículos pré-antrais disponíveis, a vitrificação tem se destacado por ser um método rápido, de baixo custo e prático, cuja eficiência tem sido confirmada na literatura pelo nascimento de descendentes saudáveis em humanos (KAWAMURA et al., 2013; SUZUKI et al., 2015), camundongos (DELA PEÑA et al., 2002) e ovinos (BORDES et al, 2005) após o autransplante do tecido ovariano. No entanto, o transplante de tecido ovariano apresenta alguns inconvenientes, tanto para a reprodução humana, como para a reprodução animal, sobretudo em espécies animais de produção. No que concerne à medicina reprodutiva humana, em alguns países da Europa e Estados Unidos, a criopreservação de tecido ovariano tem sido indicada e aplicada para mulheres jovens com câncer que irão submeter-se a tratamentos radio/quimioterápicos. Essa estratégia, após o autotransplante do ovário permite a restauração dos níveis normais de gonadotrofinas (FSH e LH) em um período de 3 a 6 meses, bem como da função reprodutiva (DONNEZ; DOLMANS et al., 2011). Entretanto, alguns autores alertam para os riscos de reintrodução de células cancerosas nas pacientes após o implante ovariano (MEIROW et al., 2008; DOLMANS et al., 2010). Já para a medicina veterinária ou animal, o transplante de órgãos e tecidos pode ter uma baixa relação custo/benefício, em função dos elevados custos dessa técnica, da necessidade de imunossupressão no caso de alotransplantes (transplante de órgãos entre indivíduos diferentes de uma mesma espécie), bem como do estresse que pode ser gerado para o próprio animal transplantado, o qual poderá interferir negativamente na função reprodutiva. Visando contornar os inconvenientes acima mencionados, a equipe do Laboratório de Manipulação de Oócitos e Folículos Pré-Antrais (LAMOFOPA) tem criopreservado folículos préantrais (secundários) com o intuito de cultivá-los, posteriormente, in vitro até à maturação oocitária para obtenção de embriões. Estudos realizados em ovinos têm mostrado que folículos secundários frescos cultivados in vitro podem resultar na produção de embriões (LUZ et al., 2012). No entanto,
24 2 a utilização de folículos secundários, previamente vitrificados, com essa finalidade, não era conhecida nesta espécie. Além disso, também não se sabia a resposta desses folículos, quando vitrificados em duas formas, isolada ou inclusa no tecido ovariano, no que se refere à obtenção de oócitos maduros após o cultivo in vitro. Portanto, uma investigação cuidadosa que envolveu aspectos morfológicos e funcionais de folículos secundários frescos e vitrificados pode indicar com segurança a melhor forma de vitrificação folicular, vislumbrando a preservação da função reprodutiva de fêmeas. Para uma melhor compreensão do tema investigado nesta tese, a revisão de literatura a seguir abordará a morfofisiologia do ovário dos mamíferos, a biotécnica de manipulação in vitro de oócitos oriundos de folículos pré-antrais, a criopreservação e o cultivo in vitro desses folículos e seus avanços, bem como as técnicas de avaliação folicular após criopreservação. Será mostrada ainda a contribuição científica desse estudo para a criobiologia e criopreservação do material genético de fêmeas.
25 3 2 REVISÃO DE LITERATURA 2.1 O ovário dos mamíferos: cenário da oogênese e da foliculogênese O ovário dos mamíferos é composto, de uma forma geral, por muitos tipos de células diferenciadas, que conjuntamente trabalham para promover um ambiente ideal para o desempenho de suas funções, endócrina e gametogênica. O ovário é composto, na maioria das espécies, por uma região interna ou medular, que consiste de tecido conjuntivo fibroelástico, tecido nervoso e vascular; e por uma região cortical, localizada na parte mais externa, circundada pelo epitélio germinal, o qual contém folículos ovarianos e corpos lúteos em vários estágios de desenvolvimento ou de regressão (LIU et al., 2006; MOHAMMADPOUR, 2007). O folículo é a unidade morfológica e funcional do ovário mamífero, cuja função é proporcionar um ambiente ideal para o crescimento e maturação do oócito, bem como produzir hormônios (MCGEE et al., 2000). O folículo ovariano é constituído por um oócito envolto por células somáticas (granulosa e/ou tecais) e circundadas pela membrana basal. O folículo ovariano é uma estrutura resultante dos processos de oogênese e foliculogênese que ocorrem no ovário ao longo da vida reprodutiva da fêmea. A oogênese consiste na formação e diferenciação do gameta feminino, e inicia-se ainda na vida fetal, quando as células germinativas primordiais (CGP) migram do saco vitelínico para as gônadas primitivas. Após sofrerem sucessivas mitoses, transformam-se em oogônias, mitoticamente ativas e, então, em oócitos primários, os quais se encontram na fase de prófase I da meiose I. A progressão da divisão meiótica ocorre somente na puberdade, com a liberação do pico préovulatório do hormônio folículo estimulante (FSH) e do hormônio luteinizante (LH) (MCGEE et al., 2000); e somente após a fecundação do oócito pelo espermatozoide, com a formação do oócito haploide fecundado, a oogênese é concluída (FIGUEIREDO et al., 2008). A foliculogênese corresponde ao processo de formação, crescimento e desenvolvimento folicular. Esse processo tem início quando células somáticas planas, isto é, as células da prégranulosa, circundam os oócitos primários, formando os folículos primordiais (JUENGEL et al., 2002) que, em ovinos, apresentam um diâmetro de aproximadamente 25 µm (MOHAMMADPOUR, 2007). Após a formação dos folículos primordiais, as células da prégranulosa param de se multiplicar e entram em um período de quiescência. Nesta categoria folicular, o núcleo do oócito é relativamente grande e ocupa uma posição central e excêntrica mostrando seu nucléolo evidente. As organelas são uniformemente distribuídas no citoplasma, das quais as mitocôndrias são as mais evidentes e podem ser arredondadas ou alongadas. Poucos complexos de Golgi são observados e ocasionalmente associados a vesículas, mitocôndrias e retículos endoplasmáticos lisos. Os
26 4 retículos endoplasmáticos lisos e rugosos estão associados a mitocôndrias e vesículas ou mesmo isolados no citoplasma. As células da pré-granulosa possuem núcleo em formato irregular que abrange grande parte do citoplasma (ANDRADE et al., 2001; MATOS et al., 2004). Durante toda a vida da fêmea, um pequeno grupo de folículos primordiais é gradualmente estimulado a crescer, constituindo a etapa de ativação folicular. O primeiro sinal de ativação dos folículos primordiais é a retomada da proliferação das células da granulosa, o que pode acontecer dias, meses ou anos após a sua formação. Uma vez ativados, os folículos entram em um curso préprogramado de desenvolvimento e maturação que são necessários para o sucesso da ovulação e fertilização, ou, alternativamente, são perdidos por atresia (MCGEE et al., 2000). Destaque-se que, apenas aproximadamente, 0,1% dos folículos primordiais chegam à ovulação (NUTTINCK et al., 1993). Após a ativação, quando uma camada completa de células da granulosa de formato cuboide circunda o oócito, são formados os folículos primários. Na espécie ovina, os folículos primários possuem diâmetro médio de 39,96 µm (MOHAMMADPOUR, 2007) e sua formação pode ocorrer ainda no período gestacional. Nesta fase do desenvolvimento folicular, além do núcleo do oócito encontrar-se em uma posição excêntrica, as organelas começam a mover-se para a periferia. O número de retículos endoplasmáticos lisos aumenta e a grande maioria das mitocôndrias apresentase alongada. À medida que os folículos crescem, as proteínas que constituem a zona pelúcida começam a ser sintetizadas (LEE, 2000). A multiplicação das células da granulosa dos folículos primários leva à formação de várias camadas destas células ao redor do oócito, dando origem aos folículos secundários, que possuem diâmetro que variam de 150 a 700 µm (LUNDY et al., 1999, PRAVEEN CHAKRAVARTHI et al., 2015). Neste estágio, a zona pelúcida é claramente identificada ao redor do oócito (PARROTT; SKINNER, 1999) e inicia-se a formação das células da teca externa a partir do estroma intersticial. Já as células da teca interna são definidas quando os folículos apresentam quatro ou mais camadas de células da granulosa. Ressalte-se que os folículos secundários são também observados em ovários de fetos ovinos (VAN DEN HURK; ZHAO, 2005). Com o crescimento dos folículos secundários e organização das células da granulosa em várias camadas, ocorre a formação de uma cavidade repleta de líquido denominada antro. A partir deste estágio, os folículos passam a ser denominados de folículos terciários ou antrais, cujo diâmetro folicular aumenta acentuadamente devido ao crescimento do oócito, multiplicação das células da granulosa, da teca e aumento do fluido na cavidade antral (DRIANCOURT, 1991). Pequenos folículos antrais podem ser similares aos folículos secundários quanto ao diâmetro (~200 µm), mas eles aumentam rapidamente em tamanho com o contínuo acúmulo de fluido folicular. Os grandes folículos antrais geralmente possuem diâmetro acima de três milímetros e contêm células da granulosa diferenciadas em células do cumulus e células murais e muitas camadas de células tecais (BRISTOL-GOULD; WOODRUFF, 2006).
27 5 Até meados da década passada, em primatas e ruminantes acreditava-se que a população folicular era estabelecida na vida intrauterina, durante a formação ovariana, ou após um curto período pós nascimento, em roedores. Esse fato estabeleceu o conceito do estoque finito e não renovável de células germinativas que tem sido considerado um princípio básico da fisiologia da reprodução há mais de 150 anos (BUKOVSKY et al., 2006). Porém, uma modificação singular do conceito de foliculogênese, com potenciais reflexos em todo o segmento da reprodução, mostrou indícios da continuidade da oogênese e foliculogênese no período pós-natal, pela atuação de células-tronco, dando origem a neofoliculogênese (CLARK et al., 2004; JOHNSON et al., 2005). Em 2004, estudos sobre a neofoliculogênese se tornaram significativos quando célulastronco embrionárias humanas cultivadas in vitro foram diferenciadas em células germinativas (CLARK et al., 2004). No ano posterior, camundongas foram esterilizadas, por quimioterapia com doxorrubicina, apresentando ausência de folículos ovarianos, porém após esses animais receberem transplante de medula óssea e sangue, folículos ovarianos viáveis e em crescimento apareceram na região ovariana (JOHNSON et al., 2005). Um estudo realizado em fetos suínos, também demostrou indícios da neofoliculogênese com a produção de estruturas semelhantes a folículos em crescimento, capazes de produzir estrógeno, progesterona e responsivas a estímulos de FSH e LH a partir da diferenciação de células-tronco de epiderme cultivadas in vitro. Além disso, os autores relataram partenogênese espontânea, com formação de estruturas semelhantes a embriões (DYCE et al., 2006; DYCE et al., 2011). Independente da possibilidade de formação de novos folículos ovarianos acorrer após o nascimento, à população folicular é bastante variável entre as espécies e, seu número é reduzido com o avançar da idade por um processo de atresia como descrito no item abaixo. 2.2 Atresia folicular Em ovelhas férteis e sadias, o número de folículos ovarianos pode variar de (CAHILL et al., 1979) a (DRIANCOURT, 1991). Vários fatores, como a idade (MONNIAUX et al., 1997), o estado reprodutivo (ERICKSON et al., 1986), a raça (CAHILL et al., 1979) e a genética (SMITH et al., 1993) podem influenciar a população de folículos ovarianos. Entretanto a maioria (cerca de 90%) dos folículos ovarianos, não ovulam, pois degeneram por um processo denominado atresia folicular, a qual ocorre por via degenerativa necrótica ou apoptótica. A morte celular por necrose, conhecida como uma morte celular passiva ocorre, geralmente, como consequência de estresse fisioquímico extremo, como: calor, choque osmótico, estresse mecânico, congelamento-descongelamento e altas concentrações de peróxido de hidrogênio (KRYSKO et al., 2008), isquemia ou restrição de nutrientes (MIKKELSEN et al., 2001). Esse tipo de morte celular é caracterizado morfologicamente pelo aumento do volume das células,
28 6 desorganização do citoplasma, disfunção mitocondrial, colapso de organelas e perda da integridade da membrana plasmática. Consequentemente, ocorre a ruptura da célula com liberação de seu conteúdo para o meio extracelular, causando dano às células vizinhas e uma reação inflamatória no local (ZONG; THOMPSON, 2006). A perda folicular por apoptose ou morte celular programada é um processo determinado geneticamente, assim, dependente,dentre outros fatores, da expressão de genes pró (BAX, BID, BIK e etc.) e anti-apoptóticos (BCL2, BCL-XL e etc.). É morfologicamente caracterizada pela condensação da cromatina (picnose celular), fragmentação específica do ácido desoxirribonucleico (DNA), perda de volume celular e formação de protuberâncias na membrana plasmática e de corpos celulares condensados, conhecidos como corpos apoptóticos (GROSSMANN, 2002). Estudos indicam que a degeneração de folículos pré-antrais em ovários ovinos, obtidos logo após a morte do animal varia de 18% a 20% (COURBIERE et al., 2005; LUNARDI et al., 2012) podendo atingir até 30% (BAUDOT et al., 2007). Apesar de ser um fenômeno natural, a atresia reduz significativamente o número de oócitos que seriam ovulados, reduzindo, portanto, o potencial reprodutivo do animal. 2.3 A biotécnica de manipulação de oócitos inclusos em folículos ovarianos pré-antrais (MOIFOPA) Durante a foliculogênese existe uma grande perda folicular que ocorre naturalmente in vivo. Portanto, a disponibilidade de oócitos é um fator limitante para o desenvolvimento de novas técnicas reprodutivas (SMITZ; CORTVRINDT, 2002). Os métodos atuais para a produção in vitro de embriões dependem de uma oferta escassa de oócitos competentes provenientes de grandes folículos antrais ou pré-ovulatórios, os quais estão presentes no ovário em número reduzido (TELFER, 1998). Desta forma, uma alternativa seria lançar mão da biotécnica de manipulação de oócitos inclusos em folículos pré-antrais (MOIFOPA), os quais são encontrados em maior quantidade no ovário, além de serem menos susceptíveis à atresia. A MOIFOPA consiste no isolamento, conservação (resfriamento ou criopreservação) e/ou cultivo in vitro de folículos pré-antrais, visando a estocagem, ativação, crescimento e maturação in vitro, com vistas à maximização do potencial reprodutivo de uma fêmea, notadamente em animais de produção. Na reprodução humana, a MOIFOPA pode ter importante relevância clínica, uma vez que possibilita o desenvolvimento de estratégias alternativas para o restabelecimento a fertilidade em mulheres com risco de falha ovariana precoce, principalmente naquelas submetidas à terapia oncológica (FIGUEIREDO et al., 2008). Apesar dessa grande perspectiva representada pela MOIFOPA, até o presente momento não se encontra disponível um protocolo de cultivo que garanta o crescimento completo de folículos e maturação de oócitos in vitro. Portanto, o que se pode admitir
29 7 é a preservação de folículos pré-antrais, para utilização futura quando um sistema de cultivo in vitro estiver plenamente estabelecido. Os tópicos abaixo descrevem os processos de criopreservação e cultivo in vitro de folículos pré-antrais, ressaltando os principais resultados obtidos com a aplicação de ambos os procedimentos, com destaque para a espécie ovina Criopreservação de folículos ovarianos pré-antrais A criopreservação, por definição, consiste na preservação de material biológico a baixas temperaturas, geralmente em nitrogênio líquido ( 196 C) ou em sua fase de vapor (~150 C negativos) por tempo indeterminado (JAIN; PAULSON, 2006). O processo de criopreservação pode ser realizado pelos métodos de congelamento lento ou vitrificação. O primeiro tem como característica principal a utilização de baixas (1 M a 3 M) concentrações de agentes crioprotetores (ACP) associada à redução gradual da temperatura (0,3 a 10 C/min). No entanto, é muito comum a formação intracelular de gelo, o qual é um dos maiores fatores responsáveis por danos à membrana plasmática (ACKER; MCGANN, 2003). Do contrário, na vitrificação a velocidade de redução da temperatura é brusca (aproximadamente C / min), que associada a altas concentrações de agentes crioprotetores, permite a passagem da água do estado líquido para um estado vítreo amorfo, sem a formação de cristais de gelo (RUBINSKY, 2003; JAHROMI et al., 2010). Além disso, a vitrificação é um método prático e de custo reduzido, o que facilita seu emprego a campo. Em virtude dessas vantagens a técnica de vitrificação foi o método de escolha na presente tese, portanto, será descrita e relatada com maior riqueza de detalhes. No processo de vitrificação, a transição para o estado vítreo ainda não está bem elucidada, porém sabe-se que o líquido super-congelado mantém as propriedades físicas de um líquido até que a temperatura de vitrificação seja atingida. Dessa maneira, o líquido adquire propriedades físicas de um sólido, mas o arranjo molecular desorganizado é mantido (WOWK, 2000), obtendo-se uma substância com características semelhantes ao estado líquido e outras próprias de um sólido cristalino. Para a obtenção da vitrificação, é necessária uma alta viscosidade da solução de vitrificação, o que é obtido pela presença de elevada (aproximadamente 6 M) concentração de ACP, e rápida redução da temperatura (WOWK, 2000). Essa combinação de fatores pode causar menos danos para o material biológico como os folículos pré-antrais. Os folículos pré-antrais podem ser criopreservados in situ (inclusos no tecido ovariano) ou isolados do ambiente ovariano. A criopreservação do tecido ovariano apresenta vantagens, pois independe da idade e fase do ciclo estral (SHAW et al., 2000), além de envolver menos questões éticas e sociais comparada à criopreservação de oócitos e embriões, sobretudo quando esse processo é realizado na espécie humana (ZHANG et al., 2009). Essa característica é extremamente
30 8 interessante para a reprodução clinicamente assistida, especialmente para mulheres que necessitam iniciar de imediato o tratamento contra o câncer (ZHOU et al., 2010). A criopreservação de tecido ovariano também é uma alternativa para meninas que ainda não tenham atingido a puberdade ou mulheres que não possuam parceiros para a doação de gametas masculinos. O estroma ovariano é muito rico em células, as quais estão justapostas. Portanto, a formação de gelo no espaço intercelular pode danificar facilmente as células e quebrar as comunicações intercelulares, necessárias para retomar o funcionamento adequado do tecido pós-criopreservação (SUGIMOTO et al., 2000). Portanto, uma alternativa no sentido de reduzir as injúrias causadas pela formação intracelular de gelo seria a criopreservação de folículos pré-antrais isolados do ambiente ovariano. Na espécie ovina, por exemplo, alguns estudos já foram reportados na literatura com a vitrificação de folículos pré-antrais inclusos ou isolados do tecido ovariano, como mostrado a seguir Avanços na vitrificação de folículos pré-antrais ovinos Embora os estudos com vitrificação de folículos pré-antrais ainda sejam pouco descritos, em 2002, dois pesquisadores do departamento de ciência animal do Iémen e de Oregon EUA mostraram que o percentual de oócitos maturados in vitro após a vitrificação de tecido ovariano foi semelhante ao de oócitos provenientes de tecido fresco ou não vitrificado (AL-AGHBARI; MENINO, 2002). Três anos mais tarde outro estudo com a espécie ovina mostrou que a viabilidade e morfologia de folículos primordiais foram mantidas após a vitrificação de ovários inteiros (COURBIERE et al., 2005), cujo resultado havia sido relatado apenas em ovários murinos (MIGISHIMA et al., 2003). Em ovelhas, até meados da década anterior, dois importantes estudos abordando o autotransplante ortotópico de tecido ovariano foram relatados na literatura (BORDES et al., 2005; LORNAGE et al., 2006). O primeiro estudo utilizou seis animais, cujos hemi-ovários tiveram a sua medula eliminada e, os fragmentos foram vitrificados e subsequentemente transplantados. Quatro meses após o transplante, foi detectada a retomada da função endócrina em todos os animais e, três ovelhas pariram quatro cordeiros após monta natural (BORDES et al., 2005). No ano seguinte, Lornage et al., (2006), investigaram os efeitos da vitrificação sobre fragmentos de ovário e ovário inteiro, bem como as propriedades físicas envolvidas na formação de cristais de gelo. Nesse estudo, os autores concluíram que a vitrificação de ovários inteiros em uma solução composta por dimetilsulfóxido (DMSO), propanodiol, polietilenoglicol e acetamida, é importante para a preservação da morfologia folicular. Essa equipe também obteve três gestações a partir das quais, quatro cordeiros nasceram depois do transplante de fragmentos do córtex ovariano previamente vitrificado.
31 9 Embora a equipe de Bordes e Lornage já tenha relatado o nascimento de crias viáveis em ovinos após o transplante de tecido ovariano vitrificado, como descrito anteriormente, a relação custo/benefício dessa prática (transplante) em animais de produção é pouco favorável. Além disso, a necessidade de imunossupressão dos animais é um fator estressante, como já mencionado anteriormente. Portanto, o cultivo in vitro de folículo pré-antrais, previamente criopreservados visando a maturação oocitária, fecundação e produção de embriões in vitro pode ser uma estratégia valiosa na reprodução assistida. Contudo, apesar dos relatos a cerca da produção de embriões de animais de produção (bubalinos: GUPTA et al., 2008; caprinos: SARAIVA et al., 2010; MAGALHÃES et al, 2011) e nascimento de crias viáveis de camundongos (EPPIG; SCHROEDER, 1989; LENIE et al., 2004), a partir de folículos pré-antrais cultivados in vitro, a eficiência dessa técnica ainda é muito baixa. Assim, como a criopreservação de folículos pré-antrais, o cultivo in vitro desses folículos ainda são considerados técnicas de reprodução assistida experimentais ou em desenvolvimento. Apesar disso, a associação de protocolos de criopreservação a sistemas de cultivo in vitro de folículos pré-antrais pode representar uma excelente estratégia para reverter ou reduzir o impacto da perda folicular. Uma vez que os sistemas de cultivo folicular estejam estabelecidos, após, a criopreservação e crioestocagem, folículos pré-antrais poderão ser cultivados in vitro para a recuperação de oócitos maduros, aptos à fecundação e consequentemente a obtenção de embriões saudáveis. Esses embriões poderão ser transplantados e garantir o nascimento de indivíduos a partir de folículos pré-antrais. Quando sistemas de cultivo in vitro de folículos pré-antrais estiverem plenamente desenvolvidos, a função reprodutiva de fêmeas de espécies animais de alto valor genético, em vias de extinção ou mesmo de fêmeas humanas, poderá ser preservada. No capítulo I, desta tese é mostrada uma abordagem ampla de como a vitrificação de tecido ovariano de ovelha pode ser um modelo para o estudo da preservação da fertilidade em mulheres Cultivo in vitro de folículos pré-antrais O cultivo in vitro de folículos pré-antrais, também conhecido como ovário artificial, tem como objetivo assegurar a sobrevivência e o crescimento folicular por meio da multiplicação e diferenciação das células da granulosa, bem como, garantir a maturação oocitária in vitro (FIGUEIREDO et al., 2008). Folículos pré-antrais podem ser cultivados inclusos (cultivo in situ) ou fora (cultivo de folículos isolados) do tecido ovariano. No cultivo in situ, pode-se utilizar o ovário inteiro ou fragmentos do córtex ovariano. Nesse sistema é possível investigar principalmente os fatores que afetam a ativação de folículos primordiais quiescentes, bem como o desenvolvimento folicular (FORTUNE, 2003) até o estágio de folículos secundários (SILVA et al., 2004). Entretanto, nesse tipo de cultivo os folículos não conseguem crescer até à fase pré-ovulatória, sendo, portanto,
32 10 utilizado para este fim, o cultivo de folículos isolados. Nesse tipo de sistema, normalmente, são utilizados folículos secundários, os quais podem ser cultivados utilizando o sistema bidimensional ou tridimensional. No primeiro, os folículos são colocados diretamente sobre o suporte de plástico (placa de cultivo) ou sobre uma matriz extracelular como, o colágeno (HIRAO et al., 1994), alginato (BARRETT et al., 2010) ou fibrina-alginato (JIN et al., 2010). Já no sistema tridimensional, os folículos secundários são totalmente recobertos pela matriz, mimetizando a própria matriz extracelular ovariana (HIRAO et al., 1994). Tem sido observado grande avanço com o cultivo in vitro de folículos ovarianos pré-antrais em diferentes espécies animais, bem como na espécie humana. Estudos em camundongos mostraram que foi possível obter crias vivas a partir de oócitos oriundos de folículos pré-antrais cultivados in vitro (O BRIEN; PENDOLA; EPPIG, 2003; HASEGAWA et al., 2006). Em animais de produção como os caprinos (SARAIVA et al., 2010; MAGALHÃES et al., 2011), bubalinos (GUPTA et al., 2008), suínos (WU et al., 2001) e primatas não humanos (XU et al., 2011; 2013), o cultivo in vitro de folículos secundários resultou na produção de oócitos maduros, os quais, após fertilização in vitro, foram produzidos embriões. Já em humanos (ROY; TRACY, 1993), bovinos (GUTIERREZ et al., 2000; ITOH et al., 2002) e cães (BRASIL SERAFIM et al., 2010), folículos pré-antrais isolados cultivados in vitro se desenvolveram somente até o estágio antral. Em ovinos, o primeiro embrião proveniente de oócitos oriundos de folículos pré-antrais foi relatado após o cultivo in vitro por seis dias, em meio meio de cultivo para tecido com bicarbonato (TCM199B) suplementado com fatores de crescimento (IGF-I e TGFb), hormônios tetraiodotironina (T4), hormônio do crescimento (GH) e FSH por Arunakumari et al., (2010). No LAMOFOPA a adição do Fator Inibidor de Leucemia (LIF), na concentração de 50 ng/ml, foi crucial para a obtenção de embriões partenogenéticos a partir de folículos secundários isolados (LUZ et al., 2012). Em um recente estudo associando a criopreservação e o cultivo in vitro, a equipe do LAMOFOPA reportou um elevado percentual de folículos morfologicamente normais, a preservação da matriz extracelular e o desenvolvimento folicular após o cultivo do tecido ovariano por um período de sete dias (BANDEIRA et al., 2015). Contudo, as taxas de maturação oocitária e, consequentemente, de embriões produzidos a partir de oócitos provenientes de folículos pré-antrais frescos crescidos in vitro ainda é insatisfatória nesta espécie. Apesar disso, o cultivo in vitro pode ser uma excelente técnica para a avaliação do tecido ovariano após criopreservação, além de favorecer o desenvolvimento dos folículos criopreservados. Esse procedimento, mesmo quando realizado por um período curto (48 h), visa suprir a necessidade celular para restaurar o metabolismo folicular, permitindo a avaliação mais precisa dos danos causados pela criopreservação (DALCIN et al., 2013). É importante salientar que folículos pré-antrais criopreservados podem apresentar diferentes requerimentos nutricionais daqueles exigidos por
33 11 folículos frescos ou não criopreservados (CASTRO et al., 2014) durante o cultivo in vitro, sendo portanto, prudente atentar para a composição do meio de cultivo utilizado Importância da composição do meio de cultivo De acordo com a literatura, os principais meios de cultivo celular utilizados para folículos ovarianos são: o meio essencial mínimo - MEM simples (MATOS et al., 2007), suas modificações - α-mem (LUNARDI et al., 2015), MEM Glutamax (TRAPPHOFF et al., 2010), dentre outros, meio de cultivo de tecido - TCM199 (ARUNAKUMARI et al., 2010), além de outros meios como o McCoy s (TELFER et al., 2008) e Waymouth (DELA PEÑA et al., 2002). Esses meios diferem entre si no que compete à composição de sais, vitaminas, minerais, aminoácidos, nucleotídeos, antioxidantes e substratos energéticos. Dentre os meios disponíveis, o α-mem e o TCM199 são os mais comumente utilizados para o cultivo de folículos pré-antrais ovinos. O α-mem é rico em sais inorgânicos, aminoácidos, vitaminas e outros componentes. Este meio foi utilizado para o cultivo in vitro de folículos pré-antrais ovinos, frescos ou não criopreservados sendo possível a obtenção de oócitos em metáfase II (CECCONI et al., 1999) e embriões produzidos por partenogênese (LUZ et al., 2012). O α-mem possui em sua composição, os aminoácidos L-Asparagina, L-Alanil-L-Glutamina e, de uma forma geral, apresenta altas concentrações de outros aminoácidos e vitaminas. Além disso, o α-mem possui piruvato, que a fonte preferencial de nutrição do oócito. Diferentemente do α-mem, o TCM199 contém mais sais inorgânicos como o nitrato férrico (Fe(NO3)3 9H2O), Fosfato de potássio monobásico (KH2PO4) e o acetato de sódio (CH3COONa), além de concentrações mais baixas de aminoácidos, presença de hidroxiprolina e mais vitaminas como calciferol, ácido 4-aminobenzóico, piridoxina ou vitamina B6. Além dos compostos presentes nos meios de base, o desenvolvimento folicular in vitro, também necessita de outras substâncias como os hormônios e fatores de crescimento. No que concerne a aditivos como hormônios e fatores de crescimento, estudos anteriores com folículos pré-antrais ovinos mostraram os benefícios da utilização do FSH, do LIF e do Kit ligand (KL) como aditivos ao meio de cultivo in vitro (LUZ et al., 2012; 2013). Vários estudos indicam que o FSH atua positivamente no crescimento de folículos pré-antrais in vitro (bovinos: WANDJI et al., 1996; bubalinos: GUPTA et al., 2008), bem como mantém a integridade das células da granulosa (suínos: HIRAO et al., 1994, humanos: ROY; TREACY, 1993) e estimula a proliferação das células da granulosa através de fatores parácrinos como o IGF-I e ativina (VAN DEN HURK; ZHAO, 2005). Em ovinos foi demonstrado o efeito positivo da adição do FSH nos meios de cultivo folicular (CECCONI et al., 1999), e a presença do LIF do meio de cultivo resultou em um percentual de 21,43% de oócitos em MII (LUZ et al., 2012).
34 12 O LIF é uma glicoproteína que atua em diferentes células e tecidos através da ligação ao seu receptor de membrana (LIF-R) e da molécula transmembranária a glicoproteína 130 (GP130). Desta forma, o complexo LIF-LIF-R GP130 inicia uma cascata de sinalização celular que culminam com a proliferação celular, crescimento e sobrevivência folicular. Esses efeitos podem ser devido ao fato do RNAm, bem como a proteína do LIF estar presente nas células do estroma, bem como de folículos e oócitos em diferente estágio de desenvolvimento (NICOLA; BABON, 2015). O Kit Ligand, também denominado fator de células tronco (SCF) ou fator de crescimento multipotente, ou simplesmente KL, apresenta-se como uma molécula solúvel (KL-1) ou associada à membrana (KL-2), dependendo do processamento do RNAm após a transcrição (HUANG et al., 1992). O KL atua quando ligado ao receptor tirosina-quinase, conhecido com c-kit (CARLSSON et al., 2006). Esse fator é expresso nas células da granulosa e tem sido relacionado com a proliferação dessas células em todos os estágios do desenvolvimento folicular, em camundongos (MANOVA et al., 1993) humanos (HOYER et al., 2005) e ovelhas (TISDALL et al., 1997). O KL também atua na sobrevivência e crescimento do oócito em camundongos (JIN, et al., 2005) e humanos (DONEDA et al., 2002; CARLSSON et al., 2006). Estudos em camundongos (GALLOWAY et al., 2000) e ovinos (DRIANCOURT et al., 2000) mostraram que mutação no gene KL, afeta negativamente o desenvolvimento folicular e consequentemente a fertilidade de uma forma geral Avaliação folicular após criopreservação e cultivo in vitro Avaliação morfológica Características morfológicas, como sinais de atresia (picnose nuclear, por exemplo), danos citoplasmáticos, destacamento de membranas (basal e nuclear) e desorganização das células da granulosa são comumente visualizados em folículos pré-antrais inclusos no tecido criopreservado (DEMIRCI et al., 2003, SANTOS et al., 2011; LUNARDI et al., 2012; CARVALHO et al., 2014) ou mesmo em folículos isolados (VANACKER et al., 2013; LUNARDI et al., 2015) e são comumente identificados mediante análise por histologia clássica. Esta técnica apresenta custo moderado, simples execução e é amplamente utilizada como excelente ferramenta quantitativa (Figura 1a). No entanto, oferece apenas uma observação morfológica superficial da célula ou tecido. A microscopia eletrônica de transmissão também se baseia em parâmetros morfológicos, entretanto, é realizada utilizando um microscópio eletrônico, que possui um sistema de produção de imagens de altíssima resolução (0,1 nm), permitindo uma visualização detalhada de estruturas biológicas não visíveis na avaliação por histologia clássica (SALEHNIA et al., 2002). Dessa forma, a captação da interação entre elétrons e átomos presentes nas células, permite identificar mudanças ultraestruturais ocorridas após a criopreservação folicular, como por exemplo, pequenos danos em
35 13 membranas nucleares (do oócito e das células da granulosa). No entanto, essa técnica é essencialmente qualitativa e demanda maior tempo para sua execução, comparada à histologia clássica, o que limita a avaliação de um grande número de folículos (Figura 1b). Figura 1. Avaliação folicular por diferentes técnicas: (A) Micrografia de um folículo secundário na histologia clássica, (B) ultraestrutura de um folículo pré-antral, (C) folículos secundário viável marcado em verde pela calceína-am, (D) Folículo primordial corado pela técnica de AgNOR e (EH) Oócitos marcados pelo Hoechst indicando as diferentes fases do processo de maturação nuclear oocitária. O: oócito, N: nucléolo, ZP: zona pelúcida, CG: células da granulosa, GV: vesícula germinativa, GVBD: quebra da vesícula germinativa, MI: metáfase I, MII: metáfase II Avaliação da viabilidade folicular e oocitária Geralmente, uma avaliação morfológica é acompanhada de uma avaliação de viabilidade e nem sempre há uma correlação entre os resultados de ambas (SCHOTANUS et al., 1997). Como já mencionado, a criopreservação pode induzir a ruptura da membrana celular, imperceptível após análise por histologia clássica, e resultar na morte de folículos após o aquecimento (MARTINEZMADRID et al., 2004). A análise da integridade da membrana basal do folículo utilizando corantes vitais, como o azul tripan, ou marcadores fluorescentes, como a calceína-am e o etídio homodímero-1, são ferramentas importantes para mostrar a viabilidade folicular após procedimentos de criopreservação e cultivo in vitro. As sondas fluorescentes detectam simultaneamente células vivas e mortas marcadas em verde pela calceína-am (figura 1c) e em vermelho pelo etídio homodímero-1, respectivamente. A primeira sonda indica atividade da esterase intracelular, enzima ativa em células viáveis. Enquanto a segunda marca os ácidos nucléicos em células não viáveis, com ruptura na membrana plasmática (LOPES et al., 2009).
36 Avaliação da funcionalidade A funcionalidade normal dos folículos ovarianos pode ser avaliada in vitro de diferentes formas, como por exemplo, através da proliferação celular de células da granulosa, da atividade esteroidogênica, da maturação do oócito e da expressão gênica. No tocante à proliferação de células da granulosa, técnica como o AgNOR (argyrophilic proteins related to nucleolar organizer regions - proteínas argirofílicas relacionadas com regiões organizadoras de nucléolos) tem sido utilizada (Figura 1d). Essa técnica cora as regiões organizadoras de nucléolos, do termo em inglês: nucleolus organizer regions, (NORs) que são alças de DNA responsáveis por sintetizar ácido ribonucleico dos ribossomos (RNAr) durante o período que precede a divisão celular, a interfase (VANDELAER et al., 1999). É no nucléolo, localizado no núcleo celular, que há armazenamento e produção de RNAr, que ligados à várias proteínas formam os ribossomos (LEE et al., 1999). As NORs têm grande afinidade pela prata, em virtude da presença de proteínas associadas ao RNA ribossomal e, por isso, são também chamadas de AgNOR (KAKEJI et al., 1991). As proteínas coradas pela prata, precipitam, formam grumos e aparecem ao microscópio ótico como pontos pretos dentro dos nucléolos (SILVA et al., 2003). Portanto, com a técnica de AgNOR é possível quantificar as NORs de cromossomos ativos durante o ciclo celular e, o número ou área das NORs mostram essa atividade proliferativa, a partir da fase G1, até a fase S (MURRAY, 2004). Estudos anteriores utilizaram essa técnica para avaliar a proliferação de células da granulosa de folículos secundários de ratas (SILVA et al., 2003). A técnica de AgNOR também já foi utilizada para avaliar folículos pré-antrais inclusos no tecido ovariano ovino (BANDEIRA et al., 2015) e bovino (CASTRO et al., 2014) previamente criopreservados e cultivados in vitro. A atividade esteroidogênica ou produção de hormônios esteroides também tem sido amplamente utilizada para avaliar a funcionalidade do folículo após manipulações in vitro. Os hormônios esteróides são originários do colesterol por meio de uma série clássica de reações enzimáticas, o qual é transportado para a face matricial da membrana interna da mitocôndria sendo alvo da enzima citocromo P450 side chain cleavage (P450scc) nas células da teca e alvo do citocromo P450 aromatase (P450arom) nas células da granulosa (THOMSON, 1998; TAMURA et al., 2007). A P450scc converte o colesterol a pregnenolona, que, após, pode ser convertida a progesterona ou a andrógeno. O andrógeno pode ser convertido, formando androstenediona, a qual pode ser convertida no andrógeno mais ativo, a testosterona, pela enzima 17β hidroxisteróide desidrogenase (17β -HSD). Finalmente a testosterona pode ser convertida a estradiol pela ação da enzima P450arom (CONLEY; BIRD, 1997). De uma forma geral, o produto esteróide final secretado pelos folículos depende do perfil das enzimas esteroidogênicas expressas pelo mesmo. O
37 15 estradiol é requerido para a indução da expressão de receptores de hormônio luteinizante (LH) nas células da granulosa o que é um pré-requisito para a ovulação, assim, a alta atividade estrogênica associada com a alta atividade da aromatase é um bom indicador da dominância fisiológica folicular, do mesmo modo que a inibição da atividade da aromatase pode resultar na atresia folicular (BERGFELT et al., 1999). A maturação do oócito é uma forma extremamente eficaz de avaliação folicular e tem sido largamente utilizada com segurança (CECCONI et al., 1999; ARUNAKUMARI et al., 2007; ARUNAKUMARI et al., 2010; LUZ et al., 2012; LUZ et al., 2013). As divisões, bloqueio, retomada e progressão meióticas, bem como as modificações moleculares e estruturais que ocorrem durante o processo de maturação são fundamentais para a fertilização e sustentação dos estágios iniciais do desenvolvimento embrionário (CHARLIER et al., 2012). As células da granulosa produzem fatores inibitórios que interrompem as divisões meióticas no estágio de diplóteno da prófase I, caracterizada pela vesícula germinativa (VG) (Figura 1e). Nesta fase há síntese proteica, transcrição de RNA e reorganização de organelas. De uma forma geral, somente na puberdade, sob estímulos gonadotróficos ocorrerá a retomada da meiose, próximo à ovulação, que é desencadeada pela onda pré-ovulatória do hormônio luteinizante - LH (WANI et al., 2000). O LH atua nas células da granulosa, que por meio de junções gap, secretam fatores parácrinos para o oócito. Além disso, ocorre a quebra da vesícula germinativa (GVBD) (Figura 1f) (estágio no qual ocorre dissolução da membrana nuclear e condensação da cromatina) devido à interrupção da transferência de fatores inibidores da maturação ao oócito. Essa interrupção é decorrente da expansão das células do cumulus com consequente perda da comunicação intercelular entre os complexos cumulus oócitos e as células da granulosa. Após o estágio de GVBD o oócito deve passar pelos estágios de metáfase I (MI) (Figura 1g), anáfase I (AI) e telófase I (TI), completando a primeira divisão meiótica, momento em que há a formação do primeiro corpúsculo polar. Em seguida, deve ocorrer a progressão ao estágio de MII (Figura 1h), onde há a presença de cromossomos centralizados no fuso e um corpúsculo polar no espaço perivitelínico (GILCHRIST et al., 2004). A sobrevivência, o crescimento e à diferenciação celular podem ser monitorados pela alteração dos padrões da expressão gênica. A capacidade de quantificar os níveis de transcrição de genes específicos é fundamental para garantir uma maior avaliação das funções foliculares. O desenvolvimento folicular depende das conexões existentes entre os compartimentos foliculares, uma vez que essas conexões permitem a troca de pequenas moléculas como íons, glicose, segundos mensageiros, os quais são necessários para o crescimento e maturação oocitária (XU et al., 2008). Dentre as proteínas de membranas que formam essas comunicações, destacam-se as conexinas 37 (CX37) e 43 (CX43), as quais são importantes na interação entre oócito e células foliculares. A vitrificação pode levar à perda da integridade das conexões entre oócitos e as células da granulosa
38 16 que constituem as comunicações intercelulares (TANPRADIT et al., 2015), assim monitorá-las pela expressão gênica do RNA é de fundamental importância.
39 17 3 JUSTIFICATIVA A criopreservação de tecido ovariano tem sido estudada há mais de 60 anos e o sucesso do método de congelamento lento seguido do autotransplante tem sido demostrado pelo nascimento de mais 60 crianças saudáveis ao logo dos últimos 10 anos (DONNEZ; DOLMANS, 2015). Apesar do êxito, a congelamento lento oferece riscos de formação intracelular de gelo, uma das principais causas de crioinjúrias. Uma alternativa para driblar esse inconveniente é através da vitrificação, cujo método de criopreservação caracteriza-se pela redução brusca da temperatura, com a passagem do líquido para um estado vítreo amorfo, sem a formação de cristais de gelo. Além disso, a vitrificação é uma técnica prática, não exige a utilização de equipamentos sofisticados, como no congelamento lento, e já foi aplicada com sucesso para a criopreservação de tecido ovariano humano, resultando no nascimento de duas crianças após o transplante (KAWAMURA et al., 2013; SUZUKI et al., 2015). Embora, dezenas de nascimentos já tenham sido reportadas na espécie humana, a partir do transplante de tecido ovariano previamente criopreservado (congelado ou vitrificado), após o tratamento e cura de doenças como o câncer, não se pode descartar por completo o risco de reintrodução de células cancerosas, no momento do implante ovariano. Em animais de produção, como os ovinos, a limitação do uso dessa técnica (transplante de tecido ovariano) é manter um animal alotransplantado, sob um regime imunossupressor no rebanho. Portanto, para evitar esses dois inconvenientes, pode-se lançar mão do cultivo in vitro dos folículos pré-antrais inclusos ou isolados do tecido ovariano visando o crescimento, a maturação e a obtenção de oócitos viáveis para posterior fecundação e produção in vitro de embriões. Estudos realizados pela equipe do LAMOFOPA já mostraram que é possível obter embriões ovinos produzidos a partir de folículos secundários cultivados in vitro (LUZ et al., 2012). No entanto, esses dados se referem apenas a folículos frescos, não sendo disponível na literatura nenhum relato a cerca de folículos secundários vitrificados que tenham produzido embriões, fato que nos suscita uma série de questionamentos com relação a melhor forma para vitrificar os folículos secundários. Ou mesmo o melhor meio para cultivar os folículos vitrificados. A resposta para todos esses questionamentos estão sendo obtidas à medida que as investigações a cerca do assunto estão sendo são executadas, tanto no LAMOFOPA, como em outros laboratórios envolvidos na tentativa de preservação da função reprodutiva de fêmeas. Estudos anteriores têm demonstrado que folículos pré-antrais criopreservados inclusos em tecido ovariano apresentam baixas taxas de sobrevivência folicular durante o cultivo in vitro. Neste caso, acredita-se que esses folículos poderiam manter melhor a viabilidade, bem como o potencial de retomada da meiose dos oócitos, se fossem criopreservados isolados do córtex ovariano.
40 18 De acordo com estudos prévios em bovinos (CASTRO et al., 2014), folículos criopreservados apresentam requerimentos diferenciados daqueles observados para folículos frescos. Baseados nessa informação, acreditamos ser importante testar meios de cultivo com diferentes composições visando obter sucesso no crescimento e desenvolvimento de folículos secundários cultivados in vitro após vitrificação. Sabe-se que, não somente a aparência morfológica e estrutural, como também a função folicular e aspectos moleculares são essenciais para avaliar o êxito da manipulação (criopreservação e cultivo in vitro) de folículos pré-antrais in vitro. Portanto, nesse estudo várias técnicas foram aplicadas, como o intuito de conhecer e avaliar o impacto da vitrificação, seguida do cultivo in vitro de folículos secundários ovinos. Como o ideal é a utilização de folículos pré-antrais, por serem abundantes no ovário, porém como os folículos primordiais, não são muito responsivos ao cultivo in vitro, escolheu-se estudar uma categoria especifica de folículos, os secundários, que apesar de ainda serem pré-antrais já apresentam receptores para diversos fatores de crescimento. Folículos secundários são mais sensíveis ao método de congelação lenta do que os primordiais, porém suportam relativamente bem o método de vitrificação que transforma a solução de criopreservação no estado vítreo e inibe a nucleação e crescimento de cristais de gelo. A espécie ovina foi utilizada neste estudo devido a sua importância econômica para várias regiões do país como fonte proteica e preservação e utilização do material genético de ovinos de alto valor zootécnico ou mesmo de raças em vias de extinção, como a Morada Nova, variedade branca e, por ser uma espécie que pode ser estudada como um modelo translacional a espécie humana, em função da similaridade do processo de foliculogênese, bem como das características do ovário da ovelha e da mulher.
41 19 4 HIPÓTESES CIENTÍFICAS Diante do exposto, foram formuladas as seguintes hipóteses científicas: É possível recuperar folículos secundários viáveis após a vitrificação de fragmentos de ovário ovino; Folículos secundários vitrificados são capazes de crescer e se desenvolver in vitro; A forma de vitrificação de folículos secundários (isolados ou inclusos no tecido ovariano) influencia na resposta (morfologia, viabilidade, desenvolvimento e funcionalidade) ao cultivo in vitro; Oócitos oriundos de folículos secundários vitrificados e cultivados in vitro são capazes de retomar a meiose; O meio base (α-mem ou TCM199) de cultivo influencia o desenvolvimento folicular e oocitário, a partir de folículos secundários cultivados in vitro após vitrificação.
42 20 5 OBJETIVOS 5.1 Objetivo geral Identificar a melhor forma de vitrificação de folículos secundários ovinos para posterior cultivo in vitro e obtenção de oócitos maduros. 5.2 Objetivos específicos 1) Comparar duas formas (isolados ou inclusos no tecido ovariano) de vitrificação de folículos ovarianos secundários; 2) Avaliar a morfologia, ultraestrutura, viabilidade, crescimento folicular, formação de antro, proliferação de células da granulosa e atividade esteroidogênica após o cultivo in vitro de curta duração (6 dias) de folículos secundários vitrificados; 3) Analisar a integridade (não extrusão), crescimento folicular, formação de antro, atividade esteroidogênica folicular e maturação oocitária após o cultivo de longa duração (18 dias) de folículos secundários vitrificados; 4) Avaliar o meio de base (α-mem ou TCM 199) de cultivo sobre o crescimento, desenvolvimento folicular e maturação de oócitos após o cultivo in vitro de folículos secundários previamente vitrificados; 5) Investigar a expressão gênica de conexinas (CX37 e CX43) e membros da família BCL2 (BCL2 e BAX) após vitrificação e cultivo in vitro de longa duração de folículos secundários.
43 21 6 CAPÍTULO 1 Vitrificação de tecido ovariano de ovelha: um modelo para o estudo da preservação da fertilidade em mulheres Ewe ovarian tissue vitrification: A model for the study of fertility preservation in women Periódico: JBRA Assisted Reproduction (Publicado) (ISSN: ) Qualis B5
44 22 RESUMO Fertilização in vitro seguida de vitrificação de embriões é a opção de preservação da fertilidade feminina mais viável para pacientes com câncer que precisam salvaguardar sua fertilidade de forma emergencial. No entanto, a sua aplicação clínica tem várias limitações. A estimulação hormonal, que atrasa o início de terapia oncológica, é contraindicada em cânceres hormônio-sensíveis e para meninas pré-púberes. Neste contexto, a vitrificação de tecido cortical do ovário, antes do início do tratamento contra o câncer, pode ser utilizada para o posterior autotransplante ou para a maturação in vitro de folículos ovarianos. No entanto, a principal preocupação associada com o autotransplante é o risco de células malignas serem reintroduzidas na paciente, sendo este risco eliminado com a utilização de oócitos produzidos de folículos crescidos in vitro. A obtenção de tecidos ovarianos humano para a pesquisa é um desafio, assim, estudos experimentais são difíceis de executar devido a questões éticas, sendo necessário o uso alternativo de modelos animais para estudos de preservação da fertilidade. Semelhanças estruturais entre o ovário ovino e humano, bem como a dinâmica de desenvolvimento folicular ovariano, fazem a ovelha um possível modelo animal para o estudo da preservação da fertilidade feminina. Como a vitrificação de tecido ovariano possui o potencial de preservar a abundante reserva oocitária inclusa em folículos ovarianos pré-antrais, esta revisão irá descrever o progresso dos estudos de vitrificação de tecido ovariano em ovinos e em mulheres. Palavras - chave: Vitrificação. Ovelha. Folículo pré-antral. Ovário. Cultivo in vitro.
45 23 Title: Ewe ovarian tissue vitrification: A model for the study of fertility preservation in women Short title for use as running head: Vitrification of sheep ovarian tissue Franciele Osmarini Lunardi1, Casie Shantel Bass2, Marcelo Picinin Bernuci3, Roberta Nogueira Chaves4, Laritza Ferreira Lima1, Renato Félix da Silva1, José Ricardo de Figueiredo1; Ana Paula Ribeiro Rodrigues1* 1 Faculty of Veterinary Medicine, LAMOFOPA, State University of Ceara, Fortaleza, Ceara, Brazil. 2 North Dakota State University, Department of Animal Sciences, Fargo, North Dakota, USA. 3 Faculty of Medicine of Ribeirão Preto, Department of Gynecology and Obstetrics, University of São Paulo, São Paulo, Brazil. 4 Health Center, University of Fortaleza (UNIFOR), Edson Queiroz, Fortaleza, Ceará, Brazil. * Correspondence: Franciele Osmarini Lunardi, Laboratory of Manipulation of Oocytes and Ovarian Preantral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, CE, Brazil. Telephone: , fax: , lunardi.franciele@gmail.com Abstract Emergency in vitro fertilization followed by embryo vitrification is one feasible fertility preservation option for cancer patients. However, its clinical application has several limitations. Hormonal stimulation delays the initiation of oncotherapy and is actually contraindicated in hormone-sensitive cancers or for use in pre-pubertal females. Vitrification of ovarian cortical tissue prior to the start of cancer treatment could be utilized for autotransplantation or for in vitro maturation of follicles enclosed in ovarian tissue. Nevertheless, the major concern associated with autotransplantation is the risk of malignant cell re-introduction to the patient, which is absent with the use of follicular in vitro culture. Since obtaining ovarian tissues from women for research is challenging and experimental studies are difficult to complete due to ethical issues, exploring the alternative usage of animal models for fertility preservation may provide beneficial insight into the prospects of follicular culture as an alternative for fertility restoration following ovarian tissue vitrification. Similarities between the ewe and human ovary structure, as well as in ovarian follicular development dynamics, make the ewe a possible animal model for the study of female fertility preservation. As vitrification of ovarian tissue has the potential to cryopreserve preantral ovarian follicles, the present review will describe the progress of ovarian tissue vitrification studies completed in ewes. Keywords: Vitrification; ewes; preantral follicle; ovary; in vitro culture.
46 24 1. Introduction In general, the treatment indicated for women diagnosed with a malignant disease is often aggressive, and may include surgeries like oophorectomy. Additionally, other women undergo chemotherapy or radiotherapy; however, all unfortunately result in premature menopause and, consequently, infertility (Amorim et al., 2011a). Advances in assisted reproductive techniques (ART) have greatly increased the possibility of fertility preservation in women submitted to gonadotoxic treatments. The main alternatives used in clinical routine for the preservation of female fertility are protection of the ovaries against radiation (oophoropexy), emergency in vitro fertilization (IVF) and oocyte vitrification. Although oophoropexy may offer some protection to germ cells, it may also considerably reduce the chances of successful future pregnancies (Wallace et al., 2005). There are also limitations with the use of IVF in cancer patients, as the hormone stimulation protocols needed to obtain mature oocytes delay the beginning of cancer treatment. These hormonal treatments are also not recommended to young pre-pubertal patients or to adult women without a partner (Wallace et al., 2005). However, the damage caused to ovarian germ line cells by radioand/or chemotherapy and the time needed for hormonal therapies would not be inconveniences with the removal and vitrification of ovarian tissue biopsies prior to therapy commencement. In view of the low availability of ovarian tissue from women and of the ethical and legal aspects involved in these procedures, some animal models, such as sheep, for example, have been extensively utilized in cryopreservation studies (Gosden et al., 1994; Bordes et al., 2005), as well as in in vitro preantral follicular culture (Campbell et al., 2000). Considering the importance of protecting female fertility, especially in women submitted to gonadotoxic treatments, and the prior usage of sheep as an animal model for humans, this review will: 1) establish a parallel between women and ewes regarding folliculogenesis, and 2) discuss the progress of ovarian tissue vitrification in both species. 2. Comparison of the main ovarian features between women and ewes Women s ovaries are almond-shaped, measure 3 cm in length, approximately cm in width and are 1 cm thick. Similarly, in the ewe, the ovaries are also the approximate size of an almond (measuring approximately 1.7 cm in length, 1.2 cm in width and 1 cm thick) (Mohammadpour, 2007). The formation and differentiation of the female gamete, or oogenesis, commences while in utero, with primordial germ cells (PGC) migrating from the vitelline sac to the primitive gonads (gonadal ridge). In women, PGC form in the vitelline sac sometime after the first month of
47 25 pregnancy; in the ewe, the colonization of PGC occurs between days 18 and 28 of embryonic development (Lun et al., 1998). These cells undergo multiple mitotic divisions, populating the developing ovarian cortex. In women, these mitotic divisions occur until close to the end of the fifth fetal month (Gartner; Hiatt, 2003); in ewes, the process ends at approximately 75 days of embryonic development (Smith et al., 1993). At that time, each ovary contains five to seven million oogonia. About one million oogonia are enveloped by follicular cells and survive until the time of birth. The remaining oogonia do not form ovarian follicles (Gartner; Hiatt, 2003) but instead undergo atresia. The duration of ovarian folliculogenesis (period of follicular growth from the primordial to the preovulatory stage) takes approximately 175 days in women (Gougeon, 1986; Wallace; Kelsey, 2010) and 170 days in ewes (Cahill; Mauleon, 1980; Bartlewski et al., 2011). The primordial follicles, as well as the oocytes contained within, have similar diameters in both ewes and women, as depicted in Table 1. The oocyte nucleus is relatively large in both species and occupies a central position, clearly showing its nucleolus. In contrast, primary and secondary follicles and their corresponding oocytes are larger in sheep than in women (Table 1). Granulosa cell numbers increase, as well as in size and protein content of the oocyte, and formation of the basal lamina and zona pellucida layers denote secondary follicle formation (Van Den Hurk; Zhao, 2005). As shown in Table 1, ewe secondary follicles and their respective oocytes are larger than those of women. Secondary follicles are also observed in fetal ovaries, when the outer theca cells form from the interstitial stroma (Van Den Hurk; Zhao, 2005). In contrast, the inner theca cells are defined after the follicles possess four or more layers of granulosa cells (Lucci et al., 2001). With the continuous growth of secondary follicles and the organization of granulosa cells into various layers, a cavity filled with fluid is formed, denoted as the antrum. From this stage, follicles are termed tertiary, or antral, and their diameter increases considerably due to oocyte growth, the multiplication of granulosa cells and the increase in the fluid of the antral cavity (Mcgee; Hsueh, 2000; Bartlewski et al., 2011). With continuing growth and development, one or two antral follicles can potentially differentiate to become a Graafian, or preovulatory follicle. In response to the preovulatory gonadotropin surge occurring in each reproductive cycle, the Graafian follicle ovulates to release the mature oocyte ready to, potentially, be fertilized. However, throughout antral development, the majority of follicles undergo degeneration or atresia, the process responsible for the depletion of most of the ovarian follicles present in the ovary (Mcgee; Hsueh, 2000; Balasch et al., 2010; Bartlewski et al., 2011). 3. Sheep ovaries as a model for fertility preservation studies in women
48 26 Many variables can affect ovarian tissue viability during vitrification, such as cryoprotectant type or concentration, cell exposure duration, tissue fragment size, cooling device, and speed of cooling. These variable effects must be exhaustively evaluated to maximize current procedures. As previously mentioned, the detailed study of human female ovarian tissues is not feasible. Therefore, as a general rule, animal models should be biochemically, physiologically and anatomically comparable with human ovarian structures. For example, ovary size, stroma layer thickness, ovulations per cycle, and estrous cycle (Vandeberg, 2004; Gerritse et al., 2008). Although there is no animal model that is a perfect human model, studies completed in bovine, porcine, equine, non-human primates, and ovine have provided tremendous amounts of valuable that has been utilized in human medicine With cow ovaries being easily accessible from abattoirs, bovine physiological functions are well documented. (Lavranos et al., 1994; Yang; Fortune, 2006; Irving-Rodgers et al., 2006). However, bovine ovaries are larger than the ovaries from women and the stromal tissue is much thicker (Gerritse et al., 2008). Swine ovary size (approximately 7.3 cm3; Gerritse et al., 2008) is similar to the ovaries of women (6.5 cm3; Munn et al., 1986) but the sow is a poly-ovulatory species (Soede et al., 2011). In fact, the sow often ovulates more than a ten oocytes per estrus. In addition, the large lipid content within swine oocytes impairs proper vitrification. A detailed study conducted on both cow and sow demonstrated that the particularities in their ovarian structures make them not ideal species to be used as a model for women (Gandolfi et al., 2006). Human and mare dominant follicle follicular fluid contains greater estradiol and progesterone concentrations and lower androstenedione concentrations when compared to subordinate follicles. Similarities in follicular wave development in both species, as well as other features, have provided reasons for utilizing the mare as an additional model for studying ovarian follicular development in women (Schneyer et al., 2000; Donadeu; Ginther, 2002; Baerwald et al., 2012). Non-human primates, like Rhesus macaques, have menstrual cycles that are analogous to women in duration and both steroid and protein hormone profiles. Anatomical similarities also exist when comparing oocytes between humans and non-human primates. Yet, limits the monkey model presents must also be considered. For example, a limited number of laboratories house monkeys and there are ethical restrictions over the use of such highly sentient animals in biomedical research (Wolf et al., 2008). However, ewe ovarian cortex has structure that is similar to ovaries in women (Munn et al., 1986, Gerritse et al., 2008). Sheep, like women, have an ovarian stroma rich in collagen, mainly located around the primordial follicles. These follicles are generally found in large clusters that represent the follicular reserve pool (Arav et al., 2005). It should be emphasized that the
49 27 cryopreservation of either cortex pieces or the entire ewe ovary is being closely studied because of the extensive knowledge about their ovarian physiology. In addition, ewe ovarian endocrine function after auto-transplantation have been studied for more than 40 years (Goding et al., 1967). Because of the ovarian similarities between this species and women, the first publication of a human birth after cryopreserved ovarian tissue transplantation (Donnez et al., 2004) was based on a protocol developed and tested using sheep as a model (Gosden et al., 1994). However, it is still necessary to conduct further comparative studies of human and ewe ovaries as they are not identical and have subtle physiological differences. 4. Ovarian tissue cryopreservation Hundreds of immature oocytes inside preantral follicles are located in the ovarian cortex and can be cryopreserved in situ, i.e., enclosed within the ovarian tissue. Immature oocytes within preantral follicles are normally more resistant to cryopreservation than mature oocytes. This is due to multiple factors such as the small size of the oocyte, low metabolic rate, stage of the cell cycle (quiescent or in prophase I), as well as the fewer supporting cells, cortical granules, and the smaller quantity of intracytoplasmic lipids (Shaw et al., 2000; Kagawa et al., 2009). The advantages of ovarian cortex cryopreservation, in addition to the large number of follicles, is that the material can be obtained regardless of the age or phase of the menstrual or estrous cycle. Moreover, this process involves fewer ethical and social questions than the cryopreservation of embryos, especially when applied to the human species (Shaw et al., 2000). This characteristic is extremely pertinent for human assisted reproduction, especially for women who must start cancer treatment immediately. The ovarian tissue cryopreservation, as mentioned early, is also an alternative for girls who have not yet reached puberty or women who do not have a partner for the donation of male gametes (Wallace et al., 2005). Preantral follicle oocytes are more resistant to the process of cryopreservation in comparison to mature oocytes (Shaw et al., 2000; Kagawa et al., 2009). In fact, the transplantation of frozen-thawed ovarian tissue or the isolation of their enclosed preantral follicles for further in vitro maturation represent a promising alternative to female fertility restoration, specifically in women that require immediate therapy or to pre-pubertal girls (Woodruff et al., 2009). The transplantation of frozen-thawed tissue has been successfully utilized, with reports of 30 human births (Donnez; Dolmans, 2013). The in vitro culture of ovarian follicles may be more viable since it eliminates the possibility of reintroduction of cancer cells back into the patient (Donnez et al., 2011). Although slow freezing has been successfully utilized for several years, the technique has some disadvantages compared to vitrification (Amorim et al., 2011a). The major disadvantage is ice formation during slow freezing, which results in cell structure damage. Since vitrification uses rapid
50 28 cooling that results in solidification without crystallization, ice formation is prevented (Bagchi et al., 2008). Vitrification of ovarian tissue has been investigated in a variety of species by using of several protocols, as well as different tissue sizes (fragments, hemi-ovaries or even whole ovaries). Tissue cryopreservation is substantially different from the cryopreservation of cells in suspension, such as isolated preantral follicles. Several cell types within a tissue contribute to its complex final physiological function and the survival of each cell type after cryopreservation is critical. Ovarian stromal tissue is very rich in regards to both cell number and variety. Accordingly, extra- and intracellular ice formation can easily damage these cells, hindering cellular communication needed for the resumption of appropriate tissue function after cryopreservation (Amorim et al., 2011a). An alternative to reduce injuries caused by the ice formation would be the cryopreservation of preantral follicles isolated from ovarian tissue since this system utilizes follicles that are removed from surrounding tissue (Amorim et al., 2011a). When the follicles are cryopreserved separate from ovarian tissue, they have the advantage of facing no risks of damage caused by ischemia and revascularization and greater facility of cryoprotectant perfusion due to the absence of tissue barriers. This technique also allows for the possibility of individual follicle monitoring during the procedures of in vitro culture (Amorim et al., 2011a). Vanacker et al. (2013) evaluated the survival and growth potential of human preantral follicles isolated before and after cryopreservation. Researchers reported that human preantral follicles can be successfully cryopreserved prior to or after isolation without impairing their ability to survive and grow in vitro. Since the preservation of preantral follicles enclosed in ovarian tissue via the vitrification method have resulted in positive outcomes, (Bordes et al., 2005; Lornage et al., 2006; Wang et al., 2011) we will describe here reports concerning ewe and human ovarian tissue cryopreserved by vitrification. 5. Advances in the ovarian tissue vitrification in sheep One of the first studies with sheep ovarian vitrified tissue utilized a vitrification solution originally tested in cow oocytes using a solution consisting of ethylene glycol (EG), fetal bovine serum (FBS), polyvinylpyrrolidone and trehalose. This solution permitted the vitrification of immature oocytes in ovarian tissue, as well as their retrieval and development during in vitro maturation up to the second meiotic division. In this study, the percentage of oocytes matured in vitro after vitrification was similar to non-cryopreserved oocytes (Al-aghbari; Menino, 2002). Courbiere et al. (2005) demonstrated for the first time the possibility to maintain primordial follicle viability and morphology after whole sheep ovary vitrification, a fact that had been only demonstrated prior in mice. In that study, two different vitrification solutions (VS) were used. One denoted VS1 and the other VS4, consisting of dimethylsulfoxide (DMSO), acetamide, polyethylene
51 29 glycol, and propanodiol (PROH), or DMSO, formamide and PROH, respectively. VS1 was described by Rall and Fahy (1985) and utilized in murine embryos, while VS4 was first used for rabbit kidney vitrification (Kheirabadi; Fahy, 2000). Ewe research on sheep progressed considerably with the publication of two successful reports, both after an orthotopic autotransplant of ovarian tissue vitrified in VS1, a solution previously described by Rall and Fahy in1985 (Bordes et al., 2005; Lornage et al., 2006) (Table 2). The first of the two cited studies used six animals in which the cortical tissues were vitrified and later transplanted. Endocrine ovarian function resumption was detected in all animals four months after the transplant, with three of these ewes successfully producing offspring after natural mating (Bordes et al., 2005). In the second study, the orthotopic autotransplant of vitrified ovarian fragments resulted in three pregnancies with the production of four lambs (Lornage et al., 2006). Several other studies were conducted on the vitrification of whole sheep ovaries (Courbiere et al., 2006; 2009). Baudot et al. (2007) tested the VS4 solution previously described by Kheirabadi and Fahy (2000) for whole ovary vitrification and obtained reasonable rates of follicular viability (61.4 ± 2.2%) and maintenance of normal morphology (48 ± 3.8%). Also in 2007, Courbiere et al. demonstrated that the reestablishment of hormone production was also maintained after the transplant of whole vitrified ovaries. Fathi et al. (2011) compared vitrification techniques using two or four solutions with increasing EG and DMSO concentrations. Morphological evaluation was performed to quantify follicles in different developmental phases (primordial, primary, secondary, and antral) and differences in survival rates were observed among the different follicular classes. In general, vitrification performed with only two solutions yielded positive results with a lower incidence of cell death as evaluated by the TUNEL technique. The vitrification technique used was called cryopin since ovarian fragments are picked up with an insulin needle, immersed in liquid nitrogen and then stored in cryotubes. Although several studies have been conducted to develop a cryoprotectant, as well as a satisfactory vitrification technique (Fathi et al., 2011; Lunardi et al., 2012), until now, there has been no consensus regarding the best solution or even vitrification technique for sheep ovarian tissue. The few births reported in the literature thus far originated from ovarian tissue vitrified in the VS1 solution with direct immersion of ovarian tissue in liquid nitrogen, which were stored in cryotubes (Bordes et al., 2005; Lornage et al., 2006) Advances in the in vitro culture of vitrified sheep ovarian tissue Although the combination of vitrification and in vitro culture has resulted in the birth of mice (Hasegawa et al., 2006; Wang et al., 2011), there is no documented, similar success in sheep.
52 30 The few studies conducted so far have demonstrated that the addition of antioxidant agents like ascorbic acid, considerably improves the tissue viability after in vitro culture, both in the culture medium and in the vitrification solution. This may be due to their role in assisting collagen biosynthesis. It was also demonstrated that the vitrification solution containing EG associated with ascorbic acid promoted better results than the association with DMSO (Melo et al., 2011). The viability of ovarian vitrified tissue analyzed after 48 h of in vitro culture was similar to the fresh control with the use of a vitrification solution containing EG (6 M), fetal calf serum (FCS) (10%) and sucrose (0.25 M) (Lunardi et al., 2012). 6. Advances in the ovarian tissue vitrification in women Pioneering studies using vitrification methods were conducted on fetal ovaries obtained from elective abortions in China (Zhang et al., 1995) (Table 3). Despite relevant results obtained in this study, only years later other researchers performed vitrification in the women ovarian tissue. After the vitrification of women s ovaries, ovarian follicles and the stromal cells were better morphologically preserved than in the slow-freezing group (Chang et al., 2011). Rahimi et al. (2003) evaluated different vitrification protocols and reported that women s ovarian tissues that were cooled rapidly did not have statistically increased apoptosis compared with fresh controls. A later study demonstrated that both oocyte viability and granulosa cell proliferation was also maintained after vitrification (Kagawa et al., 2009). Although increased success has been obtained using vitrification as a viable method for cryopreserving ovarian tissue, studies have shown that follicular apoptosis (Zhou et al., 2010) and disrupted morphology (Xiao et al., 2010, Amorim et al., 2011b) are still higher than those found in fresh tissue. In women, vitrification of ovaries combined with xenotransplantation is an ample field for research, but few studies have investigated xenografting after warmed, post-vitrified human ovarian (Rahimi et al., 2004; Rahimi et al., 2009; Rahimi et al., 2010; Amorim et al., 2012). Rahimi et al. (2004) observed no increase in necrotic area proportion in the human vitrified-thawed ovarian tissue after 42 days of subcutaneous xenotransplantation in severe combined immunodeficiency (SCID) mice compared to fresh or slow human cryopreserved ovarian tissue. The same team later reported, in contrast, that xenografts of vitrified-thawed human ovarian tissue in SCID mice after 30 days had a significantly greater amount of apoptotic cells when compared to slow frozen (Rahimi et al., 2009). Angiogenesis, the development of new blood vessels from preexisting ones, is delayed after tissue transplantation, triggering ischemia and hypoxia which results in massive follicular loss until vascularization is reestablished. After human ovarian tissues were vitrified, thawed, and xenotransplantation for 30 days in SCID mice, the tissues were observed to have vascularization
53 31 similar to tissues undergoing a similar process, but previously had been frozen (Rahimi et al., 2010). Amorim et al. (2012) observed a lower percentage of DNA damage in follicles within vitrified-thawed human ovarian tissue after 7 days of xenotransplantation in SCID mice. Since, in addition to these data, a single birth after ovarian vitrification was reported in women (Kawamura et al., 2013), further research should be directed to this area to find an ideal protocol to vitrify women ovarian tissue Advances in the in vitro culture of vitrified women ovarian tissue Since the beginning of the last decade, several studies have shown that the association of vitrification and in vitro culture can be successfully applied to human ovarian tissues, promoting stroma (Keros et al., 2009) and follicle morphology (Lee et al., 2000) preservation. Follicle morphology has been evaluated after vitrified-thawed ovarian tissue in vitro culture for short or long periods (between one and 21 days) (Salehnia et al., 2012; Isachenko et al., 2003) and has been reported that is possible to preserve the morphology similar to that of fresh tissue (Salehnia et al., 2012; Isachenko et al., 2008; Lee et al., 2000; Isachenko et al., 2003), similar to slow freezing tissue (Huang et al., 2008), or even better than slow freezing tissue (Keros et al., 2009). Li et al. (2007) found no significant differences in the proportion of morphologically normal primordial and primary follicles after in vitro culture (14 days). Ultrastrutural features of the chromatin in oocytes and follicular cells were normal after vitrified-thawed ovarian tissue and in vitro culture for one day, which was similar to the fresh tissue control (Salehnia et al., 2012). These authors also demonstrated that the use of a vitrification solution based on the content of EG or a mixture of EG and DMSO did not affect primordial or primary follicle morphology after one day of tissue culture. Follicle viability has been preserved so that it is similar to that of fresh tissue after vitrifiedthawed ovarian tissue and in vitro culture for 14 days (Lee et al., 2000). Isachenko et al. (2006) showed that the in vitro culture of vitrified tissue in large volumes of culture medium (30 ml) under constant shakings guarantied an increase in the follicular viability. These results suggest that the combination of these procedures favors the maintenance of ovarian function. Estradiol and progesterone concentrations detected in the culture medium of vitrified-thawed ovarian tissue were similar to those found in slow-freeze preserved tissues after 10 (Huang et al., 2008) and 14 (Li et al., 2007) days of in vitro culture. Estradiol concentrations from slow-frozen and vitrified ovaries was similar (Oktem et al., 2011) after three days of in vitro culture, and estradiol concentrations from vitrified ovaries was similar to the fresh tissue after 21 days of in vitro culture (Isachenko et al., 2003).
54 32 Other characteristics have also been evaluated in in vitro cultured ovarian tissues after vitrification such as apoptosis, DNA fragmentation (Salehnia et al., 2012) and oocyte maturation (Zhang et al., 1995). Apoptosis was similar when compared to slow freezing tissue after ten days of in vitro culture (Huang et al., 2008). DNA fragmentation and oocyte maturation were similar to that reported in fresh tissue after one and 40 days of in vitro culture, respectively (Zhang et al., 1995). 7. Perspectives of the in vitro culture of isolated early stage ovarian follicles to produce mature oocytes for embryo production As discussed earlier, a marked follicular loss occurs naturally in vivo. Thus, the availability of oocytes is a limiting factor for new reproductive technique development and maximization. The current methods for the in vitro embryo production depends on a scarce supply of competent oocytes from large or preovulatory antral follicles, which are present in reduced numbers in the ovary (Telfer, 1998). Therefore, the use of preantral follicles, which represent 95% of the entire follicular reserve in the mammalian ovary, is an alternative to these limitations. In this regard, the development of an in vitro system that maximizes the oocyte potential of preantral follicles should be considered. The isolation, cryopreservation and complete in vitro culture of preantral ovarian follicles may yield mature oocytes that can be utilized for further ART techniques, such as IVF and cloning, resulting in a greater number of in vitro produced embryos (Figueiredo et al., 2008). In veterinary medicine, the main objective of follicular culture is to increase the productivity of high genetic value animals and to permit the preservation of species threatened with extinction. In addition, the technique represents an excellent alternative for the encouragement and support of research related to the pharmaceutical industry by means of toxicological assays of the effect of substances on the reproductive function of females (Cortvrindt; Smitz, 2002). In human medicine, follicular culture can be relevant to clinical reproduction, since it permits the development of alternative strategies for the reestablishment of fertility in women at risk of premature ovarian failure, especially those submitted to cancer treatment. In this respect, cryopreservation associated with ovarian follicle in vitro culture can represent an excellent strategy for the reversal or reduction of the impact of follicular loss. A large stock of preantral ovarian follicles can be cryopreserved and maintained at a low temperature for long periods of time before they become atretic or degenerated. These follicles could be later thawed and cultured in vitro to obtain mature oocytes, thus guaranteeing reproductive function maintenance in women. Until presently, few studies using in vitro culture of isolated preantral follicle have been performed in the human model (Xu et al., 2009a; Vanacker et al., 2013). The success of this technique has been acquired in the mouse model with the achievement of healthy offspring (Xu et
55 33 al., 2006). Promising results have been also shown in others animals such sheep (Luz et al., 2012; Luz et al., 2013) and non-human primate (Zelinski et al., 2008; Xu et al., 2009b) but further studies are required for the acquisition of increased mature oocytes for embryo production. 8. Conclusion and prospective Ovarian tissue vitrification has proved to be a valuable tool for the female fertility preservation. The in vitro tissue culture method might be strategically used to restore reproductive capacity, especially in women submitted to cancer treatment. Although promising results regarding the combination of vitrification and in vitro culture of sheep or human ovarian follicles have been reported in the literature, further studies are needed to obtain viable embryos produced in vitro. Within this context, in view of the difficult execution of experiments in the human model due to the limited material recovered from human reproduction clinics, there is a clear need to identify the most appropriate animal to be utilized as a model for human research. Based on the reports described in the present review, sheep are believed to be a valid model for this proposal. It can be used as a model not only to improve the vitrification protocols, but also to achieve success in the production of embryos from immature oocytes grown in vitro.
56 34 Table 1. Folliculogenesis features in sheep and women. Sheep Women Primordial follicles 40.8 ( ) µm 35.4 ( ) µm Oocytes from primordial follicles 34.6 ( ) µm 32.1 ( ) µm Primary follicles 75.2 ( ) µm 46.0 ( ) µm Oocytes from primary follicles 52.1 ( ) µm 32.6 ( ) µm Secondary follicles ( ) µm 77.2 ( ) µm Oocytes from secondary follicles 72.9 ( ) µm 47.8 ( ) µm Ovaries length, width and thick 1.7 x 1.2 x 1 cm 3 cm x x 1 cm Folliculogenesis duration Approximately 170 days More than 200 days References Lundy et al., 1999; Gougeon; Chainy, 1987 Lundy et al., 1999; Gougeon; Chainy, 1987 Lundy et al., 1999; Gougeon; Chainy, 1987 Lundy et al., 1999; Gougeon; Chainy, 1987 Lundy et al., 1999; Gougeon; Chainy, 1987 Lundy et al., 1999; Gougeon; Chainy, 1987 Mohammadpour, 2007; Gartner; Hiatt, 2003 Cahill; Mauleon, 1980; Gougeon, 1986
57 35 Table 2. Advances in sheep ovarian tissue vitrification. Reference Tissue Vitrification device Vitrification solution Main outcomes Al-aghbari; Menino, Bordes et al., Ovarian fragments (0.5cm 0.5 cm). Ovarian fragments (1 mm x 2 cm x 1 cm). Tissue dropped on the surface of a steel cube cooled by LN2. Cryogenic vials plunged directly into LN2. 35% EG, 5% polyvinylpyrrolidone, 0.4 mol/l trehalose and 20% FBS. Maintenance of oocyte recovered rate and percentage of oocytes developing to metaphase. Pregnancies occurred after ovarian cortex autotransplantation, 4 lambs were born. Courbiere et al., Whole ovaries with vascular pedicle. Whole ovaries. Samples plunged directly into LN2. Courbiere et al., Lornage et al., Ethyl vinyl acetate cryobag plunged into LN2. Cryotube plunged into LN2. Ovarian fragments (1 mm thick and 1 cm2 surface). Baudot et al., Whole ovary with Ethyl vinyl acetate blood vessels. cryobag plunged into LN2. Courbiere et al., Fathi et al., Whole ovary with Ethyl vinyl acetate blood vessels. cryobag plunged into LN2. Ovarian Cryopin (sample fragments (1 x 2 adhered in a needle) 2.62 mol/l DMSO, 2.60 mol/l acetamida, 1.31 mol/l 1.2 PROH, and mol/l PEG (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution) mol/l DMSO, 2.76 mol/l formamide, and 1.97 mol/l PROH (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution) mol/l DMSO, 2.76 mol/l formamide, and 1.97 mol/l PROH (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution) mol/l DMSO, 2.60 mol/l acetamida, 1,31 mol/l 1.2 PROH, and mol/l PEG mol/l DMSO, 2.76 mol/l formamide and 1.97 mol/l PROH (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution) mol/l DMSO, 2.76 mol/l formamide, and 1.97 mol/l PROH (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution). 60% HTCM, 15% EG, 15% DMSO, 0.25 mol/l SUC and 10% HSA. Maintenance of primordial follicle viability comparable to the fresh tissue. Maintenance of the aspect of the ovarian vein identical to the nonvitrified control. Pregnancies occurred after autotransplantation of vitrified warmed ovarian cortex and lambs were born. Maintenance of ovarian primordial follicle density and follicle membrane integrity similar to the fresh tissue. One sheep recovered ovarian endocrine function 6 months after transplantation. Maintenance of intact antral follicles using 2-step dehydration protocol.
58 36 Melo et al., Lunardi et al., Torre et al., x 2 mm3). Ovarian fragments (1 mm3). Ovarian fragments (3 x 3 x 1: 9 mm3). Whole ovaries. plunged into LN2. Solid-surface plunged 40% EG, 0.5 mol/l SUC and 50 µg/ml AA. into LN2. Macrotube plunged into LN2. Ethyl vinyl acetate cryobags plunged into LN2. 6 mol/l EG, 0.25 mol/l SUC and 10% FCS. Maintenance of follicular viability rates similar to the fresh tissue after 5 days of tissue culture. Maintenance of follicular viability similar to the cultured non-vitrified tissue after 2 days of tissue culture. Significant reduction in ovarian pedicle metabolism after vitrification mol/l DMSO, 2.76 mol/l formamide and 1.97 mol/l PROH (gradual dehydration in: 12.5%, 25%, 50% and 100% of vitrification solution). Note: LN2 = Liquid nitrogen, EG = Ethylene glycol, FBS = Fetal bovine serum, DMSO = Dimethylsulfoxide, PROH =: Propylene glycol, PEG = Polyethylene glycol, HTCM = HEPES tissue culture medium, SUC = Sucrose, HSA = Human serum albumin, AA = Ascorbic acid, FCS = Fetal calf serum.
59 37 Table 3. Advances in women ovarian tissue vitrification. Reference Tissue Vitrification device Zhang et al., Fragments of fetal ovary (0.5 1 mm3). Ovarian fragments (0.8 mm x 0.8 mm x 0.8 mm). Ovarian fragments (1 ± 0.5 mm3). Plastic cryo straws directly 4.2 mol/l DMSO, 0.35 mol/l SUC Maintenance of oocyte quality similar to the plunged into LN2. and 15 mg/ml BSA. fresh tissue after 40 days of tissue culture. Ovarian fragments ( mm). Ovarian fragments (1 mm3). Ovarian fragments (1 x 1 x 5 mm). 25% Glycerol, 25% EG, 15% FCS and No rise in the proportion of necrotic areas 1% Supercool X-100 (last solution after 42 days of xenotransplantation from three steps). compared to fresh or slow cryopreserved tissue mol/l EG, 2.82 mol/l DMSO and The addition of DMSO to the vitrification 20% FCS. solution reduced primary follicles cryoinjuries. 20% DMSO, 40% EG and 10% SSS Morphologically normal follicles were (last solution from two steps). observed when vitrified ovarian tissue was cultured for 14 days in a large volume of culture medium in combination with agitation. 2 mol/l DMSO, 2 mol/l PROH, 0.2 Maintenance of estradiol and progesterone mol/l SUC and 12% HSA. production during 14 days of tissue culture similar to the slow-frozen tissue. Isachenko et al., Rahimi et al., Rahimi et al., Gandolfi et al., Isachenko et al., Li et al., Ovarian fragments (5 x 1 x 1 mm). Vitrification solution Main outcomes Straws or grids directly plunged into LN2. 40% EG, 0.35 mol/l SUC and 5% egg Maintenance of the proportion of yolk extract. morphologically normal follicles similar to the fresh tissue. Straws, grids or metal filings directly plunged into LN2 or into nitrogen vapour. Straws directly plunged into LN2. 40% EG, 0.35 mol/l SUC and 10% Cooling using nitrogen vapour resulted in egg yolk extract or 40% EG, 18% significantly elevated ROS levels and Ficoll and 0.35 mol/l SUC. apoptosis after warming. Straws directly plunged into LN2. Cryovials directly plunged into LN2. Minimum drop size directly plunged into LN2.
60 38 Huang et al., Ovarian fragments (5 x 1 x 1 mm). Solid-surface vitrification. Isachenko et al., Ovarian Droplet directly plunged fragments (about into LN2. 1 mm3). Wang et al., Ovarian fragments (~ mm2). Ovarian fragments (1 x 10 x 10 mm). Ovarian fragments (1 x 1-2 x 5-8 mm). Ovarian fragments (~ 0.5 x 1 x 1 mm). Needle directly plunged into LN2. Xiao et al., Ovarian fragments (~ 2-3 mm2). Needle directly plunged into LN2. Zhou et al., Ovarian fragments (1 x 1 x 1mm). Direct cover vitrification (DCV) or conventional vitrification (CV). Kagawa et al., Keros et al., Rahimi et al., % DMSO, 20% EG, 25 mg/ml HSA (gradual dehydration in: 25%, 50%, 75% and 100% of vitrification solution) mol/l DMSO, 2.6 mol/l acetamide, 1.31 mol/l PROH and mol/l PEG (gradual dehydration in: 25%, 50%, 75% and 100% of vitrification solution). 15% EG, 15% DMSO and 0.5 mol/l SUC. Metal strip plunged into LN2 (Cryotissue) 20% EG, 20% DMSO, 0.5 mol/l SUC. Cryo straws (Hand-cut straw). 1.4 mol/l DMSO, 1.5 mol/l EG, 1.5 PROH, 10 mg/ml HSA, and 10% PVP (last solution from three steps) mol/l DMSO, 2.6 mol/l acetamide, 1.31 mol/l PROH and mol/l PEG (gradual dehydration in: 25%, 50%, 75% and 100% of solution) mol/l EG, 1.69 mol/l DMSO and 0.5 mol/l SUC (last solution from two steps). Droplet directly plunged into LN2. 15% EG and 15% DMSO or 20% EG and 20% DMSO. Maintenance of percentage of intact primordial follicles and secretion of estradiol and progesterone within 10 days of tissue culture similar to the slow-frozen tissue. Maintenance of normally developed follicles similar to the fresh tissue after 12 days of tissue culture. Maintenance of ultrastructure of the stromal cells better than the slow-freezing or the dropping vitrification group.. Maintenance of oocyte viability similar to the fresh tissue. Preservation of ovarian stroma morphology after 1 day of tissue culture better than slow freezing procedure. Maintenance of revascularization of ovarian tissue similar to the frozen tissue after 30 days of xenotransplantation. The use of needle immersed vitrification method allowed the use of lower cryoprotectant concentration leading to improvement in the tissue cryopreservation. DCV showed a higher percentage of normal follicles and promoted less apoptotic cells compared with CV.
61 39 Amorim et al., 2011b. Salehnia et al., Ovarian fragments (1 mm3). Ovarian fragments (1 1.5 mm3). Oktem et al., Ovarian fragments (0.25 cm). Amorim et Ovarian al., fragments (1.0 x 1.0 x 1.0 mm). Droplet directly plunged into LN2. 38% EG, 0.5 mol/l trehalose, 6% FBS in MEM-GlutaMAX at 10%. Maintenance of high percentage of normal follicles than solid-surface vitrification. Cryovials or cryovials precooled to 0 C put in nitrogen vapour for 30 s and then immersed LN2. 40% EG, 30% Ficoll, 1 mol/l SUC and 1.2% BSA or increasing concentrations (2.5%, 5% and 10%) of DMSO, PROH and EG with 10% HSA. Tissue loaded into vials and immersed in LN2. 15% PROH, 15% EG, 0.2 mol/l SUC and 10% HSA. Solid-surface vitrification or open cryo straws. 20% DMSO, 20% EG and 25 mg/ml HSA or 10% DMSO, 26% EG, 2.5% PVP, 20 mg/ml HSA and 1 mol/l SUC. Maintenance of proportions of normal follicles, DNA fragmentation and ultrastructural characteristics similar to the fresh tissue after 1 day of ovarian cortex culture. Maintenance of estradiol production similar to the slow-frozen tissue after 3 days of tissue culture. Percentage of follicles with DNA damage lower than in the slow-frozen tissue after 7 days of xenotransplantation. Kawamura Pregnancy and birth et al., 2013 Note: LN2 = liquid nitrogen, DMSO = Dimethylsulfoxide, SUC = Sucrose, BSA = Bovine serum albumin, EG = Ethylene glycol, ROS = Reactive oxygen species, FCS = Fetal calf serum, PVP = Polyvinylpyrrolidone, FBS = Fetal bovine serum, PROH = Propanediol, SSS = Serum substitute supplement, HSA = Human serum albumin, PEG = Polyethylene glycol.
62 40 9. Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review 10. Funding This work was supported by CAPES. 11. References Al-aghbari, A.M., A.R. Menino (2002) Survival of oocytes recovered from vitrified sheep ovarian tissues. Animal Reproduction Science 71(1-2): Amorim, C.A., A. David, A. Van Langendonckt, M.-M. Dolmans, J. Donnez (2011b) Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertility and Sterility 95(3): Amorim, C.A., M. Curaba, A. Van Langendonckt, M.-M. Dolmans, J. Donnez (2011a) Vitrification as an alternative means of cryopreserving ovarian tissue. Reproductive Biomedicine Online 23(2): Amorim, C.A., M.-M. Dolmans, A. David, J. Jaeger, J. Vanacker, A. Camboni, J. Donnez, A. Van Langendonckt (2012) Vitrification and xenografting of human ovarian tissue. Fertility and Sterility 98(5): Arav, A., A. Revel, Y. Nathan, A. Bor, H. Gacitua, S. Yavin, Z. Gavish, M. Uri, A. Elami (2005) Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Human Reproduction 20(12). Baerwald, A.R., G.P. Adams, R.A. Pierson (2012) Ovarian antral folliculogenesis during the human menstrual cycle: a review. Human Reproduction Update 18(1): Bagchi, A., E.J. Woods, J.K. Critser (2008) Cryopreservation and vitrification: recent advances in fertility preservation technologies. Expert Review of Medical Devices 5(3): Balasch, J. (2010) Ageing and infertility: an overview. Gynecological Endocrinology 26(12): Bartlewski, P.M., T.E. Baby, J.L. Giffin (2011) Reproductive cycles in sheep. Animal Reproduction Science 124(3-4): Baudot, A., B. Courbiere, V. Odagescu, B. Salle, C. Mazoyer, J. Massardier, J. Lornage (2007) Towards whole sheep ovary cryopreservation. Cryobiology 55(3): Bordes, A., J. Lornage, B. Demirci, M. Franck, B. Courbiere, J.F. Guerin, B. Salle (2005) Normal gestations and live births after orthotopic autograft of vitrified-warmed hemi-ovaries into ewes. Human Reproduction 20(10):
63 41 Cahill, L.P., P. Mauleon (1980) INFLUENCES OF SEASON, CYCLE AND BREED ON FOLLICULAR-GROWTH RATES IN SHEEP. Journal of Reproduction and Fertility 58(2): Campbell, B.K., E.E. Telfer, R. Webb, D.T. Baird (2000) Ovarian autografts in sheep as a model for studying folliculogenesis. Molecular and Cellular Endocrinology 163(1-2): Chang, H.J., J.H. Moon, J.R. Lee, B.C. Jee, C.S. Suh, S.H. Kim (2011) Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. Journal of Obstetrics and Gynaecology Research 37(8): Cortvrindt, R.G., J.E.J. Smitz (2002) Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Human Reproduction Update 8(3): Courbiere, B., A. Baudot, V. Odagescu, B. Salle, C. Mazoyer, J. Massardier, J. Lornage (2006) Physical experimental studies to improve a vitrification procedure in whole sheep ovaries permeated with cryoprotectant agents. Human Reproduction 21: I107-I107. Courbiere, B., J. Massardier, B. Salle, C. Mazoyer, J.F. Guerin, J. Lornage (2005) Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertility and Sterility 84: Courbiere, B., L. Caquant, C. Mazoyer, M. Franck, J. Lornage, B. Salle (2009) Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertility and Sterility 91(6): Courbiere, B., L. Caquant, C. Mazoyer, M.T. Poirel, M. Franck, J. Lornage, J.F. Guerin, B. Salle (2007) Ovarian endocrine function recovery after vitrification and transplantation of whole sheep ovary. Human Reproduction 22: I227-I227. Donadeu, F.X., O.J. Ginther (2002) Changes in concentrations of follicular fluid factors during follicle selection in mares. Biology of Reproduction 66(4): Donnez, J., M.-M. Dolmans (2013) Fertility preservation in women. Nature Reviews Endocrinology 9(12): Donnez, J., M.M. Dolmans, D. Demylle, P. Jadoul, C. Pirard, J. Squifflet, B. Martinez-Madrid, A. Van Langendonckt (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364(9443): Donnez, J., S. Silber, C.Y. Andersen, I. Demeestere, P. Piver, D. Meirow, A. Pellicer, M.M. Dolmans (2011) Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Annals of Medicine 43(6): Fathi, R., M.R. Valojerdi, H. Eimani, F. Hasani, P.E. Yazdi, Z. Ajdari, L.S. Tahaei (2011) SHEEP OVARIAN TISSUE VITRIFICATION BY TWO DIFFERENT DEHYDRATION PROTOCOLS AND NEEDLE IMMERSING METHODS. Cryoletters 32(1): Figueiredo, J.R., A.P.R.Rodrigues, C.A. Amorim, J.R.V. Silva (2008) Manipulação de Oócitos Inclusos em Folículos Ovarianos Pré-antrais. In: Gonçalves, P.B.D., Figueiredo, J.R., Freitas, V.J.F. Biotécnicas Aplicadas à Reprodução Animal São Paulo: Editora Roca,
64 42 Gandolfi, F., A. Paffoni, E.P. Brambilla, S. Bonetti, T.A.L. Brevini, G. Ragni (2006) Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertility and Sterility 85: Gartner, L.P., J.L. Hiatt (2003) Tratado de histologia: Introdução à Histologia e Técnicas Básicasde Histologia. Edição 2. São Paulo: Guanabara, 472 pages. Gerritse, R., C.C.M. Beerendonk, M.S.L. Tijink, A. Heetkamp, J.A.M. Kremer, D.D.M. Braat, J.R. Westphal (2008) Optimal perfusion of an intact ovary as a prerequisite for successful ovarian cryopreservation. Human Reproduction 23(2). Goding, J.R., McCracke.Ja, D.T. Baird (1967) STUDY OF OVARIAN FUNCTION IN EWE BY MEANS OF A VASCULAR AUTOTRANSPLANTATION TECHNIQUE. Journal of Endocrinology 39(1). Gosden, R.G., D.T. Baird, J.C. Wade, R. Webb (1994) RESTORATION OF FERTILITY TO OOPHORECTOMIZED SHEEP BY OVARIAN AUTOGRAFTS STORED AT -196-DEGREESC. Human Reproduction 9(4): Gougeon, A. (1986) DYNAMICS OF FOLLICULAR-GROWTH IN THE HUMAN - A MODEL FROM PRELIMINARY-RESULTS. Human Reproduction 1(2): Gougeon, A., G.B.N. Chainy (1987) MORPHOMETRIC STUDIES OF SMALL FOLLICLES IN OVARIES OF WOMEN AT DIFFERENT AGES. Journal of Reproduction and Fertility 81(2): Hasegawa, A., N. Mochida, T. Ogasawara, K. Koyama (2006) Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertility and Sterility 86: Huang, L.L., Y.Q. Mo, W.J. Wang, Y. Li, Q.X. Zhang, D.Z. Yang (2008) Cryopreservation of human ovarian tissue by solid-surface vitrification. European Journal of Obstetrics Gynecology and Reproductive Biology 139(2): Irving-Rodgers, H.F., K.D. Catanzariti, W.J. Aspden, M.J. D'Occhio, R.J. Rodgers (2006) Remodeling of extracellular matrix at ovulation of the bovine ovarian follicle. Molecular Reproduction and Development 73(10). Isachenko, E., V. Isachenko, F. Nawroth, G. Rahimi, R. Kreienberg, J. Reinsberg, J. Weiss (2008) Human ovarian tissue preservation: Is vitrification acceptable method for assisted reproduction? Cryoletters 29(4): Isachenko, E., V. Isachenko, G. Rahimi, F. Nawroth (2003) Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. European Journal of Obstetrics Gynecology and Reproductive Biology 108(2): Isachenko, V., M. Montag, E. Isachenko, K. van der Ven, C. Dorn, B. Roesing, F. Braun, F. Sadek, H. van der Ven (2006) Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reproductive Biomedicine Online 13(2): Kagawa, N., S. Silber, M. Kuwayama (2009) Successful vitrification of bovine and human ovarian tissue. Reproductive Biomedicine Online 18(4):
65 43 Kawamura, K., Y. Cheng, N. Suzuki, M. Deguchi, Y. Sato, S. Takae, C.-h. Ho, N. Kawamura, M. Tamura, S. Hashimoto, Y. Sugishita, Y. Morimoto, Y. Hosoi, N. Yoshioka, B. Ishizuka, A.J. Hsueh (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proceedings of the National Academy of Sciences of the United States of America 110(43): Keros, V., S. Xella, K. Hultenby, K. Pettersson, M. Sheikhi, A. Volpe, J. Hreinsson, O. Hovatta (2009) Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Human Reproduction 24(7): Kheirabadi, B.S., G.M. Fahy (2000) Permanent life support by kidneys perfused with a vitrifiable (7.5 molar) cryoprotectant solution. Transplantation 70(1): Lavranos, T.C., H.F. Rodgers, I. Bertoncello, R.J. Rodgers (1994) ANCHORAGEINDEPENDENT CULTURE OF BOVINE GRANULOSA-CELLS - THE EFFECTS OF BASIC FIBROBLAST GROWTH-FACTOR AND DIBUTYRYL-CAMP ON CELL-DIVISION AND DIFFERENTIATION. Experimental Cell Research 211(2). Lee, S.H., C.S. Shin, J.J. Ko, H.C. Lee, C. Park, K.A. Lee (2000) In vitro culture of the human adult ovarian tissues after vitrification: comparison among detection methods of the culture effect. Fertil Steril, 74 (Suppl 1), 161. Li, Y.-b., C.-q. Zhou, G.-f. Yang, Q. Wang, Y. Dong (2007) Modified vitrification method for cryopreservation of human ovarian tissues. Chinese Medical Journal 120(2): Lornage, J., B. Courbière, C. Mazoyer, V. Odagescu, A. Baudot, A. Bordes, M. T. Poirel, M., Franck, B. Salle (2006) Ovarian tissue vitrification: cortex and whole ovary in sheep. Gynecol obstetfertil Lucci, C.M., R.V. Silva, C.A. Carvalho, R. Figueiredo, N. Bao (2001) Light microscopical and ultrastructural characterization of goat preantral follicles. Small Ruminant Research 41(1): Lun, S., P. Smith, T. Lundy, A. O'Connell, N. Hudson, K.P. McNatty (1998) Steroid contents of and steroidogenesis in vitro by the developing gonad and mesonephros around sexual differentiation in fetal sheep. Journal of Reproduction and Fertility 114(1): Lunardi, F.O., V.R. Araujo, L.R. Faustino, A.d.A. Carvalho, R.F. Braga Goncalves, C.S. Bass, S.N. Bao, K.P. Olazia Name, C.C. Campello, J.R. de Figueiredo, A.P. Ribeiro Rodrigues (2012) Morphologic, viability and ultrastructural analysis of vitrified sheep preantral follicles enclosed in ovarian tissue. Small Ruminant Research 107(2-3): Lundy, T., P. Smith, A. O'Connell, N.L. Hudson, K.P. McNatty (1999) Populations of granulosa cells in small follicles of the sheep ovary. Journal of Reproduction and Fertility 115(2): Luz, V.B., V.R. Araujo, A.B.G. Duarte, G.M. Silva, R.N. Chaves, I.R. Brito, M.K.B. Serafim, C.C. Campello, C. Feltrin, M. Bertolini, A.P. Almeida, R.R. Santos, J.R. Figueiredo (2013) Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Ruminant Research 115(1-3): Luz, V.B., V.R. Araujo, A.B.G. Duarte, J.J.H. Celestino, T.F.P. Silva, D.M. Magalhaes-Padilha, R.N. Chaves, I.R. Brito, A.P. Almeida, C.C. Campello, C. Feltrin, M. Bertolini, R.R. Santos, J.R. Figueiredo (2012) Eight-Cell Parthenotes Originated From In vitro Grown Sheep Preantral Follicles. Reproductive Sciences 19(11):
66 44 McGee, E.A., A.J.W. Hsueh (2000) Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews 21(2): Melo, M.A.P., I.C. Oskam, J.J.H. Celestino, A.A. Carvalho, S.V. Castro, J.R. Figueiredo, A.P.R. Rodrigues, R.R. Santos (2011) Adding Ascorbic Acid to Vitrification and IVC Medium Influences Preantral Follicle Morphology, but Not Viability. Reproduction in Domestic Animals 46(4): Mohammadpour, A. A. (2007). Comparative Histomorphological Study of Ovary and Ovarian Follicles in Iranian Lori-Bakhtiari Sheep and Native Goat. Pakistan Journal of Biological Sciences Munn, C.S., L.C. Kiser, S.M. Wetzner, J.E. Baer (1986) OVARY VOLUME IN YOUNG AND PREMENOPAUSAL ADULTS - US DETERMINATION - WORK IN PROGRESS. Radiology 159(3). Oktem, O., E. Alper, B. Balaban, E. Palaoglu, K. Peker, C. Karakaya, B. Urman (2011) Vitrified human ovaries have fewer primordial follicles and produce less antimullerian hormone than slowfrozen ovaries. Fertility and Sterility 95(8): Rahimi, G., E. Isachenko, H. Sauer, V. Isachenko, M. Wartenberg, J. Hescheler, P. Mallmann, F. Nawroth (2003) Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reproduction Fertility and Development 15(6): Rahimi, G., E. Isachenko, V. Isachenko, H. Sauer, M. Wartenberg, S. Tawadros, J. Hescheler, P. Mallmann, F. Nawroth (2004) Comparison of necrosis in human ovarian tissue after conventional slow freezing or vitrification and transplantation in ovariectomized SCID mice. Reproductive Biomedicine Online 9(2): Rahimi, G., V. Isachenko, P. Todorov, S. Tawadros, P. Mallmann, F. Nawroth, E. Isachenko (2009) APOPTOSIS IN HUMAN OVARIAN TISSUE AFTER CONVENTIONAL FREEZING OR VITRIFICATION AND XENOTRANSPLANTATION. Cryoletters 30(4): Rahimi, G., V. Isachenko, R. Kreienberg, H. Sauer, P. Todorov, S. Tawadros, P. Mallmann, F. Nawroth, E. Isachenko (2010) Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. European Journal of Obstetrics & Gynecology and Reproductive Biology 149(1): Rall, W.F., G.M. Fahy (1985) ICE-FREE CRYOPRESERVATION OF MOUSE EMBRYOS AT 196-DEGREES-C BY VITRIFICATION. Nature 313(6003): Salehnia, M., M. Sheikhi, S. Pourbeiranvand, M. Lundqvist (2012) Apoptosis of human ovarian tissue is not increased by either vitrification or rapid cooling. Reproductive Biomedicine Online 25(5): Schneyer, A.L., T. Fujiwara, J. Fox, C.K. Welt, J. Adams, G.M. Messerlian, A.E. Taylor (2000) Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: Relationship to circulating hormone concentrations. Journal of Clinical Endocrinology & Metabolism 85(9): Shaw, J.M., A. Oranratnachai, A.O. Trounson (2000) Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology 53(1):
67 45 Smith, P., W.S. O, N.L. Hudson, L. Shaw, D.A. Heath, L. Condell, D.J. Phillips, K.P. McNatty (1993) EFFECTS OF THE BOOROOLA GENE (FECB) ON BODY-WEIGHT, OVARIAN DEVELOPMENT AND HORMONE CONCENTRATIONS DURING FETAL LIFE. Journal of Reproduction and Fertility 98(1): Soede, N.M., P. Langendijk, B. Kemp (2011) Reproductive cycles in pigs. Animal Reproduction Science 124(3-4): Telfer, E.E. (1998) In vitro models for oocyte development. Theriogenology 49(2): Torre, A., M. Momier, C. Mazoyer, J. Selva, B. Salle, J. Lornage (2012) Validation of a new metabolic marker to assess the vascular viability of vitrified whole sheep ovaries. Human Reproduction 27(6): Van den Hurk, R., H. Zhao (2005) Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 63(6): Vanacker, J., V. Luyckx, C. Amorim, M.-M. Dolmans, A. Van Langendonckt, J. Donnez, A. Camboni (2013) Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertility and Sterility 99(5): VandeBerg, J.L. (2004) Need for primate models in biomedical research. Gynecologic and Obstetric Investigation 57(1). Wallace, W.H.B., R.A. Anderson, D.S. Irvine (2005) Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncology 6(4): Wallace, W.H.B., T.W. Kelsey (2010) Human Ovarian Reserve from Conception to the Menopause. Plos One 5(1). Wang, X.Q., S. Catt, M. Pangestu, P. Temple-Smith (2011) Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction 141(2): Wang, Y., Z. Xiao, L. Li, W. Fan, S.-W. Li (2008) Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Human Reproduction 23(10): Wolf, D.P. (2008) The non-human primate oocyte and embryo as a model for women, or is it vice versa? Theriogenology 69(1): Woodruff, T.K. (2009) Preserving fertility during cancer treatment. Nature Medicine 15(10): Xiao, Z., Y. Wang, L. Li, S. Luo, S.-W. Li (2010) Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertility and Sterility 94(6): Xu, M., E.R. West-Farrell, R.L. Stouffer, L.D. Shea, T.K. Woodruff, M.B. Zelinski (2009b) Encapsulated Three-Dimensional Culture Supports Development of Nonhuman Primate Secondary Follicles. Biology of Reproduction 81(3):
68 46 Xu, M., P.K. Kreeger, L.D. Shea, T.K. Woodruff (2006) Tissue-engineered follicles produce live, fertile offspring. Tissue Engineering 12(10): Xu, M., S.L. Barrett, E. West-Farrell, L.A. Kondapalli, S.E. Kiesewetter, L.D. Shea, T.K. Woodruff (2009a) In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reproduction 24(10): Yang, M.Y., J.E. Fortune (2006) Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biology of Reproduction 75(6). Zelinski, M., M. Xu, E. West, M. Lawson, L. Shea, T. Woodruff, R. Stouffer (2008) An alginate matrix supports the three-dimensional architecture of the nonhuman primate follicle and permits coordinated development of preantral follicles to small antral follicles in vitro. Biology of Reproduction: Zhang, J., J. Liu, K.P. Xu, B. Liu, M. Dimattina (1995) EXTRACORPOREAL DEVELOPMENT AND ULTRARAPID FREEZING OF HUMAN FETAL OVA. Journal of Assisted Reproduction and Genetics 12(6): Zhou, X.H., Y.J. Wu, J. Shi, Y.X. Xia, S.S. Zheng (2010) Cryopreservation of human ovarian tissue: Comparison of novel direct cover vitrification and conventional vitrification. Cryobiology 60(2):
69 47 7 CAPÍTULO 2 Folículos ovinos secundários isolados vitrificados são capazes de crescer e de formar antro após curto período de cultura in vitro Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture Periódico: Cell &Tissue Research, v. 362 (1) p , 2015, (Publicado) (ISSN: X) Qualis A1
70 48 RESUMO O risco de reintroduzir células malignas, em pacientes curadas do câncer durante o autotransplante ovariano é uma realidade, o que limita diversas aplicações clínicas. Neste contexto, o cultivo in vitro de folículos seria uma alternativa ao transplante, a fim de minimizar tais riscos. Por isso, o objetivo deste estudo foi comparar o desenvolvimento de folículos secundários vitrificados na forma isolada (sem stroma) e in situ (em fragmentos do tecido ovariano). Para isso, folículos foram primeiramente isolados de fragmentos de ovário de ovelhas sem raça definida e então vitrificados compondo o grupo Folículo Vitrificado (Folículo-Vit), ou fragmentos de tecido ovariano foram primeiro vitrificados, seguido pelo isolamento dos folículos secundários, resultando no grupo Tecido Vitrificado (Tecido-Vit). Os grupos Controle e vitrificados foram submetidos ao cultivo in vitro (6 dias) e a avaliação da morfologia e viabilidade folicular, formação de antro, diâmetros folicular e oocitário, taxa de crescimento, bem como características ultra-estruturais e de proliferação celular. O percentual de folículos morfologicamente normais e formação de antro foram semelhantes entre os três grupos. A viabilidade folicular e diâmetro oocitário foram semelhantes entre os grupos Folículo-Vit e Tecido-Vit. O diâmetro e a taxa de crescimento folicular do grupo Folículo-Vit foram semelhantes ao Controle, enquanto esses parâmetros no grupo TecidoVit foram significativamente mais baixos em comparação ao Controle. Ambos os grupos vitrificados apresentaram maior taxa de proliferação das células da granulosa comparados ao Controle. Assim, folículos secundários podem ser vitrificados com êxito antes ou depois do isolamento do tecido ovariano, sem prejudicar a sua capacidade de sobreviver e crescer durante o cultivo in vitro. Palavras - chave: Vitrificação. Fragmento ovariano. Folículo secundário isolado. Regiões organizadoras de nucléolos. Cultivo in vitro.
71 49 Title page: Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture Franciele Osmarini Lunardi, M.Sc.a, Roberta Nogueira Chaves, Ph.D.,b Laritza Ferreira de Lima, M.Sc.,a Valdevane Rocha Araújo, Ph.D.,a Ivina Rocha Brito, M.Sc.,a Carlos Eduardo Azevedo Souza, Ph.D.,c Mariana Aragão Matos Donato Ph.D.,d Christina Alves Peixoto, Ph.D.,d Andras Dinnyes, Ph.D.e Cláudio Cabral Campello Ph.D.,a José Ricardo de Figueiredo Ph.D.,a Ana Paula Ribeiro Rodrigues Ph.D.,a* *Correspondence: Franciele Osmarini Lunardi, Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, CE, Brazil. Telephone: , fax: , lunardi.franciele@gmail.com Where the work was done: Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, CE, Brazil. Financial support: This work was supported by CNPq. Franciele Osmarini Lunardi is a recipient of a grant from CAPES Brazil. In addition, Ana Paula Ribeiro Rodrigues and José Ricardo de Figueiredo are recipients of a grant from CNPq Brazil. Conflict of interest: The authors declare that there is no potential conflict of interest. Department affiliations: a Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, CE, Brazil. b Health Center, University of Fortaleza (UNIFOR), Edson Queiroz, Fortaleza, Ceará, Brazil. c State University of Ceara, Fortaleza, Ceara, Brazil. d Laboratório de Ultraestrutura, CPqAM-FIOCRUZ, Universidade Federal de Pernambuco, Recife, PE, Brasil. e Molecular Animal Biotechnology Laboratory, Szent Istvan University, Gödöllö, Hungary.
72 50 Abstract The risk of reintroducing malignant cells after ovarian graft into patients following post-cancer treatment is an obstacle for clinical applications (autotransplantation). In this context, in vitro follicle culture would be an alternative to transplantation to minimize such risks. Therefore, the aim of this study was to compare the development of secondary follicles after vitrification in isolated form (without stroma) with vitrification in in situ form (within fragments of ovarian tissue). From mixed-breed ewes, follicles were first isolated from ovarian fragments, and then vitrified; composing the Follicle-Vitrification group (Follicle-Vit) or fragments of ovarian tissue were first vitrified, followed by isolation of the follicles, resulting in Tissue-Vitrification group (Tissue-Vit). Control and vitrified groups were submitted to in vitro culture (6 days) and follicular morphology, viability, antrum formation, follicle and oocyte diameter, growth rate, ultrastructural characteristics and cell proliferation were evaluated. The percentage of morphologically normal follicles and antrum formation were similar among groups. Follicular viability and oocyte diameter were similar between Follicle-Vit and Tissue-Vit. The follicular diameter and growth rate of Follicle-Vit were similar to the Control, while Tissue-Vit was significantly lower compared to the Control. Both vitrified groups had an augmented rate of granulosa cellular proliferation compared to Control. Secondary follicles can be successfully vitrified before or after isolation from the ovarian tissue without impairing their ability to survive and grow during in vitro culture. Key words: Vitrification. Ovarian fragments. Isolated secondary follicle. Nucleolus organizer regions. In vitro culture.
73 51 1. INTRODUCTION Over the past decades, advances in cryopreservation techniques and protocols for ovarian tissue have contributed to the establishment of germplasm banks essential for the preservation of genetic material, notably from species with high economic value or potentially endangered (Liu et al., 2008; Santos et al., 2010). In addition, the association between cryotechnology and assisted reproductive techniques (ART) has become of important clinical relevance to humans, since it allows the development of alternative strategies for restoring fertility in women at risk of premature ovarian failure, especially those undergoing cancer therapies. Currently, ovarian tissue cryopreservation is a recommended alternative to preserve fertility in patients needing treatment against malignant diseases (Lee et al., 2006). In veterinary medicine, several studies have described ovarian tissue cryopreservation and reported the birth of healthy offspring after transplantation of ovarian tissue after slow freezing (Gosden et al., 1994; Salle et al., 2002, 2003; Imhof et al., 2006) or vitrification (Bordes et al., 2005; Lornage et al., 2006) in livestock animals. Compared to slow freezing, vitrification has become a recent alternative method since it is a quicker procedure, as well as less expensive (Isachenko et al., 2009). Else, it has been largely associated with tissue transplantation to restore fertility in young women facing cancer threat (Revel et al., 2011). Furthermore, healthy live births have been already obtained after fresh or frozen ovarian tissue transplantation in different species, including humans (Donnez; Dolmans, 2013). However, the risk of reintroducing cancer cells in patients is a possibility (Dolmans et al., 2010) and has limited its clinical application. Therefore, in vitro ovarian follicle culture seems to be an alternative to substitute transplantation and minimize cancer-associated risks, providing a way to harvest more mature oocytes. However, due to ethical issues, it is quite difficult to perform experiments using human tissue. Thus, advances in sheep ovarian vitrification are relevant, since it has been demonstrated anatomically and physiologically similar to the human ovary (Gosden et al., 1994; Oktay et al., 2000; Salle et al., 2002). Our previous study has already demonstrated that vitrified preantral follicles enclosed in sheep ovarian tissue showed low rates of follicular survival during in vitro culture (Lunardi et al., 2012). In fact, it has been determined as well that human follicles maintain viability following cryopreservation even after isolation from the ovarian cortex and cryopreservation (Vanacker et al., 2013). However, no studies to date evaluated the effect of vitrification and in vitro culture after isolation of sheep secondary follicles. Thus, it is necessary to verify the possibility to isolate secondary follicles from previously vitrified tissue as efficiently as from fresh tissue. Therefore, the aim of this study was to determine if isolating sheep secondary follicles before vitrification would
74 52 improve follicle quality compared to those isolated after tissue vitrification. For this matter, follicular morphology, ultrastructure, viability, hormone secretion and development have been compared, before and after vitrification and a short-term in vitro culture. 2. MATERIALS AND METHODS 2.1. Source of ovaries Ovaries (n = 48) were collected at a local abattoir from 24 adult non-pregnant mixed-breed ewes. Immediately post-mortem, under aseptic conditions, the ovaries were washed in 70% alcohol for 10 seconds, followed by two washes in HEPES buffered minimum essential medium (MEM) supplemented with 100 µg/ml penicillin and 100 µg/ml streptomycin. Each pair of ovaries was transported to the laboratory within 1 h into tubes containing 15 ml of MEM at 4 C. Unless otherwise mentioned, the chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) Experimental design At the laboratory, ovaries were stripped out from surrounding fat and fibrous tissue, and then cortex were recovered and fragmented into pieces (1 to 2 mm thick) and split into three different groups: a) Control Group (Control): secondary follicles were isolated without any previous vitrification; b) Follicle-Vitrification group (Follicle-Vit): secondary follicles were vitrified in isolated form c) Tissue-Vitrification group (Tissue-Vit): secondary follicles were vitrified within fragments of ovarian tissue, in situ form, and subsequently subjected to isolation. From all groups isolated secondary follicles were submitted immediately to viability, morphology and ultrastructural analysis or were in vitro cultured for 6 days. During in vitro culture, in addition to the above mentioned analysis, we measured the hormone secretion (progesterone and estradiol), antrum formation and growth rate Ovarian cortex fragmentation and follicle isolation The ovarian cortex was sectioned into pieces of 1 to 2 mm thickness under sterile conditions in a Petri-dish containing MEM supplemented with HEPES and antibiotics (100 µg/ml penicillin and 100 µg/ml streptomycin). Fragments obtained were transferred to another Petri-dish containing fresh medium and then visualized under a stereomicroscope (100x, Nikon SMZ 645, Tokyo, Japan). Once located, secondary follicles, with average diameter of 280 µm (range µm), were
75 53 mechanically isolated by microdissection with the aid of 26-G needle attached to 1 ml syringe. Only those follicles with the oocyte-follicle complex visible, surrounded by several layers of granulosa cells with intact basement membrane and no antral cavities were selected for this study In vitro culture of sheep secondary follicles Secondary follicles were transferred into drops of 100 µl of culture medium under mineral oil in Petri-dishes (60 x 15 mm) and cultured for 6 days (one follicle/drop) at 39 C and 5% CO 2 in air. Culture medium contained MEM alpha modification (α-mem) supplemented with 10 µg/ml insulin, 5.5 µg/ml transferrin, 5 ng/ml selenium, 2 mm glutamine, 2 mm hypoxanthine, 3 mg/ml bovine serum albumin (BSA), 50 µg/ml ascorbic acid and 50 ng/ml leukemia inhibitory factor (LIF) and 50 ng/ml Kit Ligant (KL) according Luz et al. (2013) Morphological analysis and assessment of in vitro follicular growth Before (at day 0) and after 6 days of in vitro culture, the percentage of morphologically normal follicles and follicular diameters from Control, Follicle-Vit and Tissue-Vit were determined. Follicles were classified as morphologically normal whether if they presented intact basement membrane (no extrusion of the oocyte from the follicle), bright and homogeneous granulosa and theca cells, and homogeneous oocyte cytoplasm. Follicle degeneration was recognized when the rupture of basement membrane, as well as oocytes and surrounding cells darkening, misshapen oocytes or decreased follicle diameter were observed. Follicular diameter was measured at the basement membrane (from the major and minor axes) of each follicle with the aid of an ocular micrometer inserted into a stereomicroscope (SMZ 645 Nikon, Tokyo, Japan; 100X). The average of these 2 measurements was used to determine the follicle diameter only in morphologically normal follicles. Average follicular growth rate (mean increase in follicular diameter) was calculated as follows: the diameter of morphologically normal follicles at day 6 minus the diameter of normal follicles at day 0, divided by 6. Antral cavity formation was defined as a visible translucent cavity within the layers of granulosa cells Ovarian follicles and fragments vitrification For vitrification, the solution previously defined by Bordes et al. (2005) was used as base medium, modified by the following composition: MEM HEPES supplemented with 10% fetal bovine serum (FBS), 2.60 M acetamide, 2.62 M dimethylsulfoxide, 1.31 M 1, 2-propanediol (PROH) and M polyethylene glycol (PEG). Initially, ovarian fragments (1 to 2 mm thick)
76 54 and isolated secondary follicles were exposed to different concentrations (12.5, 25, 50 and 100%) of vitrification solution. Samples were exposed to the first two concentrations for 5 minutes at room temperature; then to the next two for 15 minutes (fragments) or 5 minutes (isolated follicles) at 4 C. Following this treatment, samples (either ovarian fragments or isolated follicles) were placed on the surface of a metal cube partially submerged in liquid nitrogen. Samples were kept into liquid nitrogen (-196 C) for 1 hour Warming protocol of ovarian follicles and fragments Samples of ovarian tissue and isolated secondary follicles were removed from liquid nitrogen and underwent three baths of 5 minutes each with the solution composed by MEM HEPES plus 10% FCS and decreasing concentrations of sucrose (0.5, 0.25 and 0.0 M) in accordance with the protocol adapted from Lunardi et al. (2012) Assessment of follicle viability by fluorescence microscopy The follicles from Control (n = 60) and vitrification groups (n = 120) recovered before and after 6 days of in vitro culture, were incubated in 100 µl drops containing 2 µm of ethidium homodimer-1 and 4 µm calcein-am (Molecular Probes, Invitrogen, Karlsruhe, Germany) at 39 C for 15 minutes. Then, the follicles were washed in MEM HEPES and analyzed using a fluorescence microscope (Nikon, Eclipse 80i, Tokyo, Japan). The fluorescent signals emitted by calcein-am and ethidium homodimer were measured at 488 and 568 nm, respectively. The oocytes and granulosa cells were considered viable when the cytoplasm was positively marked by calcein-am (green) and chromatin not marked by ethidium homodimer-1 (red) Histological analysis of isolated sheep follicles Follicles were included in 0.5% alginate (FMC Biopolymers, Philadelphia, PA) and fixed in 4% Paraformaldehyde-Cacodylate-Ca2+ buffer (4% PFA, 0.1 M Sodium Cacodylate, 0.1 M Sucrose, 10 mm CaCl2, ph=7.4) overnight at 4 C. Then, the follicles were washed (twice) in Phosphate Buffered Saline (PBS) and stained with alcian blue. Samples were dehydrated by incubation with increasing concentrations of ethanol (50 to 100%). Follicles were then included in paraffin and serial 7 µm sections were cut. The blades were mounted stained with periodic acid-schiff (PAS)hematoxylin and then analyzed by light microscopy (Nikon, Japan; 400X magnification). Follicles were classified individually as morphologically normal follicles when an intact oocyte, an oocyte without a pyknotic nucleus or cytoplasmic retraction, and granulosa cells well organized in two or
77 55 more layers with no pyknotic nucleus were present. Moreover, atretic follicles were defined as those having a retracted oocyte, pyknotic nucleus, and/or disorganized granulosa cells detached from the basement membrane. In addition, oocyte diameter was recorded from edge to edge of its membrane using a microscope (Nikon eclipse 80i) equipped with an image processor (NIS-Elements; Nikon). The average of these 2 measurements was used to determine oocyte diameters Ultrastructural analysis of sheep follicles For ultrastructural evaluation, a total of 60 follicles (30 before and 30 after in vitro culture) from Control, Follicle-Vit and Vit-Iso were included in alginate (0.5%), fixed in Karnovsky s solution (4% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, ph 7.2) for 12 h at 2-8 C, and then embedded in droplets of 4% low-melting agarose. After three washes in sodium cacodylate buffer, specimens were post-fixed in 1% osmium tetroxide, 0.8% potassium ferricyanide, and 5 mm calcium chloride in 0.1 M sodium cacodylate buffer for 1 h at room temperature. Subsequently, samples were dehydrated using a gradient of acetone solutions (31100%) and then drenched in resin SPIN-PON (Sigma Company, St. Louis, MO). Semi-thin sections (1 µm) were stained with toluidine blue and then, ultra-thin sections (60-70 nm) of three follicles per treatment (Control, Follicle-Vit and Tissue-Vit before and after 6 days of culture) were contrasted with uranyl acetate and lead citrate, and examined under an electron microscope (Fei Tecnai Spirit). Ultra structural changes were observed as the density and integrity of the cytoplasmic organelles of the oocyte and granulosa cells, the degree of cytoplasmic vacuolization and nuclear, cytoplasmic, and basal membrane integrity Assessment of cell proliferation Follicle cell proliferation rate in all samples were evaluated by quantifying the number of nucleolus organizer regions (NORs) argentaffin (Ag). Isolated follicles from all groups after 6 days of in vitro culture were histologically processed as described above. For NORs marking, after reduction with potassium iodide 1%, slides containing follicles were stained with silver nitrate solution 50% in a colloid solution (2:1) in a darkroom, and counterstained with safranin 0.1%. To quantify the NORs, 30 granulosa cells from 5 follicles (150 granulosa cells/ group) from Control and each vitrification group were visualized under a light microscope (1000 x) and counted in accordance with a protocol adapted from Castro et al. (2014) Steroidogenic activity analysis
78 56 To evaluate estradiol and progesterone concentrations on day 6 of culture, the total culture medium from 40 follicles from Control and each of the vitrification groups (combined each 4 follicles being 10 samples/group) were retrieved and stored at -80 C until analysis estradiol and progesterone concentrations were determined using the ARCHITECT platform (Abbott Diagnostics, Abbott Park, IL, USA). The sensitivity to estradiol and progesterone was 2.5 pg/ml and <0.1 ng/ml, respectively, according to manufacturer s instructions. This platform uses CMIA (chemiluminescence microparticle immunoassay), and has a detection limit < 5 pg/ml to estradiol and 0.1 ng/ml to progesterone. A sample of standard medium was used as negative control. Linearity for estradiol and progesterone assays is 10 to 1000 pg/ml and 0.1 to 40 ng/ml, respectively. Intra- and inter-assay Coefficients of Variability (CV) were below 10% Statistical analysis Data for continuous variables (oocytes and follicles diameters, growth rate) were initially evaluated for homoscedasticity and homogeneity (by Bartlett s and Shapiro-Wilk tests, respectively) to confirm requirements underlying analysis of variance. Then ANOVA test was carried out according to a 3 x 2 factorial arrangement of groups with procedure (non-frozen samples, isolation followed by vitrification, vitrification followed by isolation) and time of culture (0 or 6 days) as the main effects. The model used was Yij=µ+Pi+Tj+( Pi x Tj) +eijk, where Yij = dependent variable, µ= general mean; Pi = procedure, Tj = time in culture, Pi x Tj = interaction between procedure and time, and eijk = residual error. When any main effect or interactions were significant, means were compared by Student-Newman-Keuls test, being the results expressed as mean ± standard error of the mean (SEM). Discrete variables (number of morphological normal and viable follicles, antrum formation and follicles with decreased diameter) were analyzed as dispersion of frequency by Chi-square test and results were expressed as percentages. In all cases, differences were considered to be significant when P< RESULTS 3.1. Follicle count after isolation from fresh and vitrified fragments In total from all fragments obtained from the replicate experiments (n = 282) we recovered 320 secondary follicles, among them 106, 112 and 112 originating from Control, Follicle-Vit and Tissue-Vit groups, respectively (Table 1). As shown in Table 1, the number of follicles recovered among all three groups was equivalent. It also indicates that vitrification and warming procedures
79 57 used in this study either before or after isolation do not alter significantly the yield of follicles recovered from fragments Assessment of morphologically normal follicles, antral cavity formation and follicle viability The effects of the different treatments on the percentage of morphologically normal and viable follicles as well as antrum formation are shown in Table 2. Normal follicle (Fig. 1a-e) appearing healthy with zona pellucida, follicular cells, theca cells, healthy basal membrane and spherical oocyte with uniform cytoplasm. Degenerate follicle (Fig. 1f) displaying retracted oocyte, disorganized granulosa cells, empty space in the oocyte cytoplasm. A total of 126 isolated follicles from three groups were examined. Histological analysis showed that normal (Fig. 1a-e) and degenerated follicles (Fig. 1f) were found in all groups evaluated. The results showed that both vitrification protocols were efficient to maintain follicular morphology, since at least 61.90% of morphologically normal follicles were observed after culture. Nevertheless, there was no significant difference between vitrified groups and Control (P>0.05). Similar results were found regarding antrum formation. There was no significant difference between vitrified groups (Follicle-Vit: 67.30% and Tissue-Vit: 77.41%) and Control (65.38%), respectively. Regarding follicular viability (Fig. 2), 100% of the follicles before in vitro culture, were viable in all groups. After in vitro culture, follicular viability rate was higher in the Control (100%) compared to Follicle-Vit (83.33%, P<0.05) group, and this treatment was similar to Tissue-Vit (93.33%, P>0.05) Follicle diameter and growth rate and oocyte diameter Follicular diameter, daily growth rate (µm/day) and percentage of follicles that decreased diameter during in vitro culture are shown in Figure 3, whereas the oocyte diameter is shown in Figure 4. There was a significant increase in follicular diameter from day 0 to day 6 of culture in Control and in Follicle-Vit (P<0.05). After culture, Control presented follicular diameter ( ± 14.30, Fig. 3a) and growth rate (15.99 ± 1.56, Fig. 3b) significantly higher than the Tissue-Vit ( ± 8.61 and 8.94 ± 0.72) group, respectively (P<0.05). The lowest percentage (P<0.05) of follicles with decreased diameter was observed in Control, while the highest was seen in the TissueVit (37.1%; P<0.05.) (Fig. 3c). For oocyte diameter (Fig. 4), Control was the only procedure that showed significant increase from day 0 to day 6, with significantly higher average (P<0.05) compared to vitrification treatments after 6 days of in vitro culture.
80 Ultrastructural analysis of sheep follicles Figure 5 shows micrographic pictures of the ultrastructure of follicles analyzed belonging to all three groups. Control follicles had oocyte membrane and zona pellucida well preserved. Therefore, microvilli were easily seen (Fig. 5a), although in smaller numbers after in vitro culture (Fig. 5b). In Control, mitochondria showed no apparent signs of alteration. After in vitro culture vacuolization of the cytoplasm and a slight detachment of granulosa cells have been observed. In follicles subjected to both vitrification procedures, i.e., Follicle-Vit and Tissue-Vit (Fig. 5c and 5e), oocyte membrane as well as zona pellucida were intact; however, small irregularities were visible. In these vitrification protocols, both before (Fig. 5c and 5e) and after culture (Fig. 5d and 5f) we observed a decrease in the number of microvilli, as well as in organelle density, associated with large vacuolated areas. Displacement of granulosa cells and detachment between these cells and the oocyte (Fig. 5b, d and f) was seen in follicles from all groups after in vitro culture. Particularly in Follicle-Vit, before in vitro culture, we observed good quality oocyte cytoplasm and intact zona pellucida; although granulosa cells were slightly detached from each other. After in vitro culture the vacuolization strongly increased at oocyte cytoplasm and granulosa cell levels, as well. Regarding Tissue-Vit, even prior to vitrification extensive oocyte vacuolization was observed, however, granulosa cells showed no signs of degeneration. After in vitro culture advanced oocyte degeneration was observed, together with zona pellucida irregularities, and granulosa cell detachments Cell proliferation after in vitro culture of preantral follicles To evaluate the proliferative capacity of granulosa cells of sheep follicles we used the AgNOR technique for marking of nucleolar organizer regions (Table 3). Both vitrified groups, Follicle-Vit (4.53 ± 0.14) and Tissue-Vit (4.37 ± 0.16) had a similar number of NOR and both had increased numbers compared to Control group (3.97± 0.11) (P<0.05) Steroidogenic activity analysis Control follicles released increasing amounts of estradiol (503.1 ± pg/ml), while Tissue-Vit group follicles (98.22 ± pg/ml) exhibited slight steroidogenic activity. Furthermore, for all groups, progesterone concentrations were below the detection limit of the assay (0.1 ng/ml).
81 59 4. DISCUSSION To the best of our knowledge, this is the first report of growth and antrum formation after in vitro culture of isolated ovine preantral follicles subjected to vitrification either isolated or within ovarian tissue fragments. The similar number of healthy isolated secondary follicles recovered from ovary before or after vitrification is a new result for ovine species. The recovery of isolated secondary follicles from previously vitrified ovarian cortex which were able to grow and become antral in vitro, would open promising perspectives for patients diagnosed with cancer, particularly eliminating the risk of reintroducing malignant cells after ovarian transplantation. In this case, growth and maturation of isolated ovarian follicles for fertilization and in vitro embryo production purposes are a safer assisted reproductive technology (ART) compared to transplantation of ovarian tissue in women with the desire to conceive after cancer therapy. Morphology and developmental ability to the antral cavity in secondary follicles cultured in vitro after vitrification was equivalent to the results of non-vitrified follicles. In previous, contradicting studies vitrification has been shown to cause irreversible follicular damage, resulting in inability to grow during in vitro culture (Oktem et al., 2011), while in another study (Isachenko et al., 2006) follicular morphology has been preserved after vitrification. Our results are promising, and could relate to a rich culture medium, supplemented with LIF and KL, among other additives. According to previous experiments, these substances have a positive effect on antrum formation and stimulate granulosa and theca cell proliferation (Luz et al., 2012; 2013). Additionally, these molecules can enhance embryo production from in vitro grown oocytes obtained from non-vitrified preantral follicles (Luz et al., 2013). In the present work, only few vitrified follicles lost viability after in vitro culture (FollicleVit: 16.67% and Tissue-Vit: 6.67%). These follicles had some granulosa cells marked by ethidium homodimer-1. In mice, isolated preantral follicles vitrified and treated with propidium iodide (PI) showed a decrease of 11% in their viability (Trapphoff et al, 2010). The viability of isolated follicles vitrified and in vitro cultured found in our study was similar to that reported for human follicles (Bian et al., 2013). In Follicle-Vit, the proportion of viable follicles decreased with time in culture compared to fresh follicles. Similar results were reported for mice follicles after 10 days in culture (Nagano et al., 2007). These results suggest that damages that were not detected initially, possibly resulting from mechanical stress caused by follicle isolation procedures as well as those inherent to cryopreservation (cryoprotectant toxicity, temperature changes, etc.) may take place and become evident during in vitro culture. Our data show that secondary follicles from Follicle-Vit had diameter and daily growth rate in vitro similar to those seen in non-vitrified, cultured follicles, similar to the results reported for
82 60 human follicles (Bian et al., 2013). On the other hand, follicles from Tissue-Vit had slower growth compared to cultured follicles not subjected to prior vitrification. Lin et al., (2008) showed that the growth rate of isolated mice follicles vitrified is higher than that of follicles vitrified while embedded in ovarian tissue. These results support the notion that tissue cryopreservation is substantially different than that of cells suspended in medium (Vanacker et al., 2013), as is the case of isolated preantral follicles. Different cell types collaborate simultaneously in complex ways to ensure healthy tissue physiology, being therefore very important that each cell type survives the cryopreservation procedure. Individualized treatment by each cell type may be more complex and laborious procedure, but might allow the development of more efficient protocols for preservation of ovarian follicles in different stages of development. The percentage of follicles with decreased diameter was higher in Tissue-Vit compared to Control or Follicle-Vit. Additionally, oocyte diameter in follicles from Tissue-Vit showed retraction during culture, as opposite to follicles from Follicle-Vit which kept their oocyte diameter during all 6 days of culture. Oocyte growth is also important for follicular development, since it is related to the accumulation of proteins, mrna, transcription factors and other substances necessary to the formation of the meiotic fuse and restart of meiosis (Liu et al., 2006). The method of follicular vitrification, i.e., isolated or included in ovarian tissue possibly affects its permeability to cryoprotectants through somatic cells. In the case of follicles belonging to Follicle-Vit, cryoprotectants need to cross only the membranes of granulosa and theca cells, and finally reach the oocyte. However, in the follicles from Tissue-Vit, beyond granulosa and theca cells, there are stromal cells and extracellular matrix, and these components render the perfusion process slower and more complex, potentially resulting in worse oocyte protection during all steps of the cryopreservation procedure. Similar events are present in oocytes showing loss of water and cryoprotectant penetration, resulting in differential shrinkage and morphological changes according to the vitrification protocol (Van den Abbeel et al., 2007). In terms of ultrastructure, fresh and non-cultured follicles had oocyte membrane and zona pellucida regular and intact, and visible microvilli, with a decrease in the number of these microvilli after in vitro culture. Microvilli are projections of plasma membrane essential for the communication between the oocyte and surrounding cells (granulosa or cumulus cells). These structures develop with zona pellucida during growth of the secondary follicle, allowing the exchange of metabolites essential for the development of oocyte competence (Lucci et al., 2001; Duarte et al., 2012). Vitrification can cause profound ultrastructural changes in these microvilli (Fuku et al., 1995), especially when the vitrified follicle is further subjected to in vitro culture, even for a short period (Bian et al., 2013).
83 61 The ultrastructure analyses revealed obvious damage in the oocyte and the granulosa cells of follicles vitrified in both forms (vitrified without or within fragments of ovarian tissue), although the classical histology observations seemed normal in most vitrified follicles. In general, prior to the in vitro culture period, Follicle-Vit preserved the oocyte cytoplasm better than the granulosa cells. Conversely, in the Tissue-Vit advanced oocyte degeneration has been visible, but the granulosa cells did not show such alterations. Possibly the granulosa cells from this group (Tissue-Vit) were better protected within the ovarian tissue (stroma) upon vitrification, but the penetration of the cryoprotectants may not be ideal compared to Follicle-Vit thus apparent cryoprotectant toxicity damage in the oocytes have been observed. The present work showed for the first time the cell proliferation rate using nucleolus organizing regions (NORs) as parameters in antral follicles previously vitrified and cultured in vitro. NORs are deoxyribonucleic acid (DNA) pockets responsible for signaling to ribosomal ribonucleic acid (rrna) during interphase (Silva et al., 2003). Generally, non-histone proteins have a binding site to rrna and have affinity for silver; therefore they precipitate forming nucleolar grains. That way, active chromosomes are marked during cell cycle, and the number of NORs reflects this proliferative activity. This approach had been successfully reported in previous work to measure cell proliferation rates in different rats ovarian structures (Silva et al., 2003). In the present study, NORs were well distributed in granulosa cell nuclei of antral follicles. We recorded that vitrified follicles showed increased cell proliferation rates compared to fresh follicles. It is possible that these follicles have increased proliferation rate during in vitro culture as a means to compensate potential damages related to cryopreservation. We observed a similar pattern of cell proliferation within both groups of vitrified follicles, irrespective of vitrification protocol (Follicle-Vit or TissueVit). Steroidogenic activity of secondary follicles cultured in vitro resulted in low, almost undetectable concentrations of progesterone. Cecconi et al., (2004) reported that in up to 6 days of culture estradiol production is low, increasing progressively until day 10. Follicles belonging to Control showed an increased estradiol production, numerically, compared to vitrified follicles. These results are in agreement to those reported by Cecconi et al. (2004), showing that fresh ovine follicles produce higher amounts of estradiol compared to cryopreserved follicles. Still according to these authors, estradiol production by cryopreserved follicles can reach that of fresh follicles after a longer culture time, as shown, usually from days 6 to 10. However, lower estradiol production by follicles with good viability rates (64.76%) and increases in diameter (28.90 ± 2.21 μm), have also been reported in rats for isolated secondary follicles after solid-surface vitrification (Xing et al., 2010). The Tissue-Vit viability was similar to that of the Control and ultrastructural evaluation revealed only minor damage in granulosa cells after vitrification, coinciding with a higher estradiol production than in the Follicle-Vit group. The Follicle-Vit viability was lower than that of the
84 62 Control, part of the internal theca and granulosa cells have been damaged, possibly by cryoprotectant toxicity. The ultrastructural evaluation revealed damage immediately after vitrification. In this group the estradiol level was very low, probably due to damage to internal theca and granulosa cells, which both acts together in the production of estradiol. Follicular vitrification prior to the start of the cancer treatment could be used for further autotransplantation. Nevertheless, the major concern associated with autotransplantation is the risk of re-introduction of malignant cells back to the patient. This risk is absent if they use the technique of in vitro follicle culture. Since women ovarian tissue for research is scarce, and most of the experimental studies are difficult, and often unethical to conduct, exploration of alternative animal models to study women fertility preservation might be useful to provide insights into the prospects of follicle culture as an alternative to restore fertility following ovarian tissue vitrification. Similarities in the dynamics of follicle development and ovary structure between women and sheep favor this animal model for the study of female fertility preservation. We conclude that secondary follicles can be either vitrified in isolated form or within fragments of ovarian tissue. However, isolated follicles display a better follicular growth rate and fewer follicles with decreased diameter after in vitro culture.
85 63 Fig. 1 Histological sections showing normal (a e) and degenerated (f) sheep follicles cultured for 6 days in Control (a, b) after Follicle-Vit (c, d) and Tissue-Vit (e, f). Normal follicles appear healthy with zona pellucida, follicular cells, theca cells, healthy basal membrane and spherical oocytes with uniform cytoplasm. Degenerate follicles display retracted oocytes, disorganized granulosa cells, and empty space in the oocyte cytoplasm. O oocyte, N oocyte nucleus, gc granulosa cells, ZP zona pellucida, TC thecal cells, A antrum.
86 64 Fig. 2 Assessment of the viability of sheep secondary follicles cultured for 6 days using fluorescent probes. Tissue-Vit (a, b) and Follicle-Vit (c, d) classified as viable since cells were labeled with calcein-am (green fluorescence; Image b) and non-viable as cells were marked with ethidium homodimer-1 (red fluorescence; Image d) respectively. Note follicle containing peripheral granulosa cells labeled with ethidium homodimer-1 (d).
87 65 Fig. 3 Ovine follicle diameter (µm) (mean ± SEM) (a), daily growth rate (µm/day) (mean ± SEM) (b) and the percentage of follicles with decreased diameter (c) before (day 0) and/or after 6 days of culture. A,B,C Different uppercase letters indicate statistically significant differences (P<0.05) among treatments. a,b Different lowercase letters indicate statistically significant differences (P<0.05) between days of culture..
88 66 Fig. 4 Ovine oocyte diameters (µm) (mean ± SEM) of follicles from Control, Follicle-Vit and Tissue-Vit before or after 6 days of culture. A,B Different uppercase letters indicate statistically significant differences (P<0.05) among treatments. a,b Different lowercase letters indicate statistically significant differences (P<0.05) between days of culture.
89 67 Fig. 5 Electron micrographs of ovine follicles. Different columns show follicles from Control (a-b), as well as from treatments Follicle-Vit (c-d) and Tissue-Vit (e-f), before (a, c and e) or after in vitro culture (b, d and f). In a (Control non-cultured), ultrastructural characteristics are well preserved, including regular and intact oocyte membrane and zona pellucida. Additionally, microvilli are visible in the zona pellucida. In b (Control cultured), microvilli are still visible, although in smaller numbers. Secretion vesicles shown in increased size and numbers compared to those from noncultured control. In both controls, intact organelles were visible, especially mitochondria. After vitrification (c-f), small irregularities in the zona pellucida and increased number of cytoplasmic vacuoles could be seen in follicles subjected to all treatments. In both vitrification and cultured treatments there was a small decrease in the size and numbers of the microvilli between oocyte and zona pellucida, and also low density of organelles and cytoplasmic material (d-f). ZP: zona pellucida; O: oocyte; gc: granulosa cells.
90 68 Table 1. Number of fragments and follicles recovered from Control, Follicle-Vit and Tissue-Vit in each repetition. Repetition Ovaries Fragments Fragments used Fragments used Follicles recovered Follicles recovered Follicles recovered used in in Follicle-Vit in Tissue-Vit from Control from Follicle-Vit from Tissue-Vit fragments fragments fragments Control Total Table 2. Percentages of morphologically normal, viable (calcein-am and ethidium homodimer-1) and antrum formation in sheep follicles in Control, Follicle-Vit and Tissue-Vit before and/or after 6 days of culture. Treatments Morphologically normal follicles Day 0 Day 6 Aa Aa 80.95% (17/21) Antrum formation Day 0 100% Aa (30/30) Day 6 100% Aa 67.30% A (35/52) 61.90% Follicle-Vit 66.66% Aa (14/21) 71.42% Aa (15/21) 100% Aa (30/30) 83.33% Bb (25/30) 65.38% A (34/52) Tissue-Vit 61.90% Aa (13/21) 66.66% Aa (14/21) 100% Aa (30/30) 93.33% ABa (28/30) % A (48/62) Different uppercase letters indicate statistically significant differences (P<0.05) among groups (rows). statistically significant differences (P<0.05) between days of culture (columns). a,b (30/30) Day 6 Control A,B (13/21) Viable follicles Different lowercase letters indicate
91 69 Table 3. Average number of development NORs per nucleus of granulosa cells in sheep follicles in Control, Follicle-Vit and Tissue-Vit after 6 days of culture. NORs per nucleus of granulosa cells Control 3.97± 0.11 b (150) Follicle-Vit 4.53 ± 0.14 a (150) Tissue-Vit 4.37 ± 0.16 a (150) a,b Significant differences (P < 0.05).
92 70 5. REFERENCES Bian J, Li T, Ding C, Xin W, Zhu B, Zhou C (2013) Vitreous Cryopreservation of Human Preantral Follicles Encapsulated in Alginate Beads with Mini Mesh Cups. Journal of Reproduction and Development 59: Bordes A, Lornage J, Demirci B, Franck M, Courbiere B, Guerin JF, Salle B (2005) Normal gestations and live births after orthotopic autograft of vitrified-warmed hemi-ovaries into ewes. Human Reproduction 20: Castro SV, Carvalho AA, Gomes Silva CM, Santos FW, Campello CC, de Figueiredo JR, Ribeiro Rodrigues AP (2014) Frozen and Fresh Ovarian Tissue Require Different Culture Media to Promote in vitro Development of Bovine Preantral Follicles. Biopreservation and Biobanking 12: Cecconi S, Capacchietti G, Russo V, Berardinelli P, Mattioli M, Barboni B (2004) In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biology of Reproduction 70:12-17 Dolmans M-M, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J (2010) Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 116: Donnez J, Dolmans M-M (2013) Fertility preservation in women. Nature Reviews Endocrinology 9: Duarte ABG, Araujo VR, Chaves RN, Silva GM, Magalhaes-Padilha DM, Satrapa RA, Donato MAM, Peixoto CA, Campello CC, Matos MHT, Barros CM, Figueiredo JR (2012) Bovine dominant follicular fluid promotes the in vitro development of goat preantral follicles. Reproduction Fertility and Development 24: Fuku E, Xia L, Downey BR (1995) Ultrastructural-changes in bovine oocytes cryopreserved by vitrification. Cryobiology 32: Gosden RG, Baird DT, Wade JC, Webb R (1994) Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196-degrees-C. Human Reproduction 9: Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J (2006) Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertility and Sterility 85: Isachenko V, Isachenko E, Weiss JM, Todorov P, Kreienberg R (2009) Cryobanking of human ovarian tissue for anti-cancer treatment: comparison of vitrification and conventional freezing. Cryoletters 30: Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, Braun F, Sadek F, van der Ven H (2006) Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reproductive Biomedicine Online 13: Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K (2006) American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology 24:
93 71 Lin TC, Yen JM, Kuo TC, Gong KB, Hsu KH, Hsu TT (2008) Comparison of the developmental potential of 2-week-old preantral follicles derived from vitrified ovarian tissue slices, vitrified whole ovaries and vitrified/transplanted newborn mouse ovaries using the metal surface method. Bmc Biotechnology 8:13 Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P (2006) Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Developmental Biology 299:1-11 Liu LJ, Xie XY, Zhang RZ, Xu P, Bujard H, Jun M (2008) Reproduction and fertility In wild-type and transgenic mice after orthotopic transplantation of cryopreserved ovaries from 10-d-old mice. Lab Animal 37: Lornage J, Courbière B, Mazoyer C, Odagescu V, Baudot A, Bordes A, Poirel MT, Franck M, Salle B (2006) Ovarian tissue vitrification: cortex and whole ovary in sheep. Gynecol Obstet Fertil 34: Lucci CM, Silva RV, Carvalho CA, Figueiredo R, Bao N (2001) Light microscopical and ultrastructural characterization of goat preantral follicles. Small Ruminant Research 41:6169 Lunardi FO, Araujo VR, Faustino LR, Carvalho AdA, Braga Goncalves RF, Bass CS, Bao SN, Olazia Name KP, Campello CC, de Figueiredo JR, Ribeiro Rodrigues AP (2012) Morphologic, viability and ultrastructural analysis of vitrified sheep preantral follicles enclosed in ovarian tissue. Small Ruminant Research 107: Luz VB, Araujo VR, Duarte ABG, Celestino JJH, Silva TFP, Magalhaes-Padilha DM, Chaves RN, Brito IR, Almeida AP, Campello CC, Feltrin C, Bertolini M, Santos RR, Figueiredo JR (2012) Eight-Cell Parthenotes Originated From In vitro Grown Sheep Preantral Follicles. Reproductive Sciences 19: Luz VB, Araujo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Serafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR, Figueiredo JR (2013) Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Ruminant Research 115: Nagano M, Atabay EP, Atabay EC, Hishinuma M, Katagiri S, Takahashi Y (2007) Effects of isolation method and pre-treatment with ethylene glycol or raffinose before vitrification on in vitro viability of mouse preantral follicles. Biomedical Research-Tokyo 28: Oktay K, Karlikaya GG, Aydin BA (2000) Ovarian cryopreservation and transplantation: basic aspects. Molecular and Cellular Endocrinology 169: Oktem O, Alper E, Balaban B, Palaoglu E, Peker K, Karakaya C, Urman B (2011) Vitrified human ovaries have fewer primordial follicles and produce less antimullerian hormone than slowfrozen ovaries. Fertility and Sterility 95: Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E (2011) Micro-organ ovarian transplantation enables pregnancy: a case report. Human Reproduction 26: Salle B, Demirci B, Franck M, Berthollet C, Lornage J (2003) Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: pregnancies, births, and histologic assessment. Fertility and Sterility 80:
94 72 Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J (2002) Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertility and Sterility 77: Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, Paris M, Schoenfeldt V, Martinez-Madrid B (2010) Cryopreservation of ovarian tissue: An emerging technology for female germline preservation of endangered species and breeds. Animal Reproduction Science 122: Silva CM, Serakides R, Nascimento EF, Nunes VA, Ribeiro AFC, Ocarino NM (2003) Quantification of the nucleolar organizer regions (NORs) as a parameter to measure proliferation of granulosa cells. Arquivo Brasileiro De Medicina Veterinaria E Zootecnia 55: Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U (2010) DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Human Reproduction 25: Van den Abbeel E, Schneider U, Liu J, Agca Y, Critser JK, Van Steirteghem A (2007) Osmotic responses and tolerance limits to changes in external osmolalities, and oolemma permeability characteristics, of human in vitro matured MII oocytes. Human Reproduction 22: Vanacker J, Luyckx V, Amorim C, Dolmans M-M, Van Langendonckt A, Donnez J, Camboni A (2013) Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertility and Sterility 99: Xing W, Zhou C, Bian J, Montag M, Xu Y, Li Y, Li T (2010) Solid-surface vitrification is an appropriate and convenient method for cryopreservation of isolated rat follicles. Reproductive Biology and Endocrinology 8:
95 73 8 CAPÍTULO 3 Folículos secundários ovinos vitrificados na forma isolada crescem e se desenvolvem in vitro melhor do que aqueles vitrificados em fragmentos de ovário Ovine secondary follicles vitrified out the ovarian tissue grow and develop in vitro better than those vitrified into the ovarian fragments Periódico: Theriogenology (Publicado) (ISSN: X) Qualis A2
96 74 RESUMO A criopreservação de folículos pré-antrais é uma técnica promissora para a preservação da fertilidade feminina. O objetivo deste estudo foi avaliar o efeito da vitrificação no desenvolvimento de folículos secundários inclusos em tecido ovariano ou isolados após microdissecção. Um importante parâmetro adicionado foi a capacidade dos oócitos maturados in vitro em retomar a meiose. Cortéx ovariano de ovelhas foi fragmentado e dividido em três diferentes grupos: a) grupo fresco (Controle): folículos secundários isolados do córtex ovariano sem prévia vitrificação; b) grupo Folículo-Vitrificado (Follicle-Vit): folículos secundários vitrificados após isolamento; c) grupo Tecido-Vitrificado (Tissue-Vit): folículos secundários vitrificados dentro de fragmentos de tecido ovariano (in situ) e subsequentemente submetidos a isolamento. Para os três grupos, os folículos secundários isolados foram submetidos ao cultivo in vitro por 18 dias. Após o folicular cultivo in vitro, complexos cumulos-oócitos (CCO s) foram coletados. Como um grupo controle adicional, grupo Crescidos In Vivo, CCO s foram coletados a partir de folículos ovarianos antrais crescidos in vivo. Todos os CCO s recuperados foram maturados in vitro e a viabilide e a configuração da cromatina foram avaliadas. Os dados foram analisados por ANOVA, e as médias foram comparadas pelo teste Student-Newman-Keuls, ou por Qui-quadrado. Diferenças foram consideradas significativas quando P<0,05. Folículos pré-antrais isolados de todos os tratamentos apresentaram morfologia normal, formação de antro e baixa degeneração folicular após cultivo in vitro. A taxa de crescimento entre os grupos Controle e Follicle-Vit não diferiu (P>0,05), e foi maior (P<0,05) do que o grupo Tissue-Vit. O percentual de folículos que decresceram seus diâmetros durante cultivo in vitro foi significativamente maior no Tissue-Vit do que no Follicle-Vit. A taxa de recuperação oocitária para folículos normais foi maior no Follicle-Vit do que no TissueVit. Além disso, a viabilidade oocitária foi menor no grupo Tissue-Vit do que nos outros tratamentos e o grupo Follicle-Vit não diferiu dos grupos Controle e Crescidos in vivo. O percentual de oócitos que retomaram a meiose não diferiu entre os grupos cultivados. Após a vitrficação, somente o grupo Follicle-Vit demonstrou oócito em metáfase I (MI). Concluímos que folículos secundários vitrificados após isolamento, apresentaram uma melhor taxa de crescimento folicular, viabilidade oocitária, percentual de oócitos que alcançaram o estágio de MI e menos folículos com decréscimo no diâmetro após cultivo in vitro.
97 75 Title: Ovine secondary follicles vitrified out the ovarian tissue grow and develop in vitro better than those vitrified into the ovarian fragments Running title: Vitrified follicles yield competent oocytes Summary: The follicular development, as well as the oocyte in vitro maturation was evaluated after two different forms of vitrification of sheep secondary follicles: vitrification in isolated form or within ovarian tissue fragments. Franciele Osmarini Lunardi1, Francisco Leo Nascimento de Aguiar1, Ana Beatriz Graça Duarte1, Valdevane Rocha Araújo1, Laritza Ferreira de Lima1, Naiza Arcângela Ribeiro de Sá1, Hudson Henrique Vieira Correia1, Sheyla Farhayldes Souza Domingues2, Cláudio Cabral Campello1, Johan Smitz3, José Ricardo de Figueiredo1, Ana Paula Ribeiro Rodrigues1 1 Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, CE, Brazil. 2 Wild Animal Biology and Medicine, Federal University of Pará, Belém, Brazil 3 Follicle Biology Laboratory, Center for Reproductive Medicine, UZ Brussel, Laarbeeklaan 101, B-1090 Brussels, Belgium.
98 76 Abstract Cryopreservation of preantral follicles is a promising technique to preserve female fertility. The aim of this study was to evaluate the effect of vitrification on the development of secondary follicles included in ovarian tissue or isolated after microdissection. An important endpoint included is the capacity of grown oocytes to resume meiosis. Sheep ovarian cortexes were cut into fragments and split into three different groups: a) Fresh group (Control): secondary follicles isolated without any previous vitrification; b) Follicle-Vitrification group (Follicle-Vit): secondary follicles vitrified in isolated form c) Tissue-Vitrification group (Tissue-Vit): secondary follicles vitrified within fragments of ovarian tissue (in situ former) and subsequently subjected to isolation. From the three groups, isolated secondary follicles were submitted to in vitro culture for 18 days. After in vitro culture, cumulus-oocyte complexes (COCs) were harvested from follicles. As an additional control group, In Vivo Grown, in vivo-grown COCs were collected from antral ovarian follicles. From all recovered COCs were matured and the chromatin configuration was evaluated. Data were analyzed by ANOVA, and the means were compared by Student-Newman-Keuls test, and by Chi-square. Differences were considered to be significant when P<0.05. Isolated preantral follicles from all treatments had normal morphology, antrum formation and low follicle degeneration after in vitro culture. The growth rate between Control and Follicle-Vit did not differ (P>0.05), and was higher (P<0.05) than for Tissue-Vit. The percentage of follicles that decreased diameter during in vitro culture was significantly higher in Tissue-Vit than the in other treatments. Recovery rate of oocytes from normal follicles was higher in Follicle-Vit than in Tissue-Vit. Furthermore, oocyte viability was lower in Tissue-Vit than other treatments and Follicle-Vit did not differ from Control and In Vivo Grown. The percentage of oocytes meiosis resuming was not different between treatments. After vitrification only Follicle-Vit showed metaphase I (MI) oocyte. We conclude that secondary follicles vitrified after isolation displayed a better follicular growth rate, oocyte viability, percentage of oocytes reaching the MI stage and fewer follicles with decreased diameter after in vitro culture. Keywords: Cryopreservation, Follicular development, Oocyte maturation, Ovary, Ovine/sheep.
99 77 1. Introduction Cryopreservation of ovarian tissue is nowadays considered a feasible option for fertility preservation. However, some authors have indicated the risk of reintroduction of cancer cells after transplantation [1-3]. Thus, the in vitro culture of isolated secondary follicles after cryopreservation is considered a reasonable alternative to avoid this and to obtain fertilizable oocytes [4]. Appropriate protocols for in vitro culture of non-cryopreserved preantral follicles had already been reported in murine, in which viable offspring were obtained [5]. Nevertheless, in nonhumane primates species [6, 7] or livestock animals, like caprine [8] and ovine [9], only a limited and variable number of embryos arising from preantral follicle oocytes cultured in vitro have been obtained until today. Although the higher results in animal models being plenty supportive, in humane species the results are not suitable, considering reports of low frequency of antrum formation of preantral follicles grown in vitro [10]. Notwithstanding, only in murine, embryos were obtained from previously cryopreserved ovarian tissue, with successful reports [11, 12]. In latter studies, vitrification was employed as the first choice method for ovary cryopreservation. Vitrification constitutes the ultra-rapid cooling of biological material to very low temperatures and the use of high concentrations of intracellular cryoprotectant agents (CPAs). These particular conditions allow a solidification, or amorphous liquid state of vitrification solution, which inhibit nucleation and ice crystals growth [13, 14]. The ovarian tissue is constitutes by different cellular types that vary in size, distribution and quantity, and by many follicular development stages cells (granulosa and theca cells) that form the barrier to the oocytes. All these structures represent a huge hurdle to an optimal permeability for CPAs and could result in difficulties to an adequate oocyte protection at liquid nitrogen temperature (-196 C). Recently, our group reported that secondary sheep follicles could be vitrified effectively, enclosed or not in ovarian cortex. After thawing, these follicles (previously isolated or isolated after ovarian fragment vitrification) are capable to form an antrum when cultured in vitro for 6 days, and to be similar to fresh non cultured follicles without vitrification [15]. However, due to the short in vitro culture that was applied, it was not possible to ascertain if oocytes enclosed in secondary follicles were capable to survive a long-term culture, needed to reach the development stage of oocyte to resume meiosis. The current study aimed to vitrify secondary follicles enclosed or isolated from of ovarian tissue and to evaluate relevant end points: post-thaw follicular survival, growth, antrum formation and hormone production after 18 days of culture as well as oocyte viability and maturation. 2. Materials and methods
100 78 This experiment was approved and performed under the guidelines of Ethics Committee for Animal Use of State University of Ceará. Unless otherwise mentioned, all chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) Source of ovaries Ovaries (n = 30) were collected at abattoirs from 15 adult mixed-breed ewes. Immediately post-mortem, under aseptic conditions, the ovaries were washed in 70% alcohol for 10 seconds, followed by two washes in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered Minimum Essential Medium (MEM) supplemented with 100 µg/ml penicillin and 100 µg/ml streptomycin. Each pair of ovaries was transported to the laboratory within 4 h into tubes containing 15 ml of MEM HEPES at 4 C Experimental design At the laboratory, ovaries were cleaned from surrounding fat and fibrous tissue and their cortexes was recovered and fragmented into pieces (1 to 2 mm thick) and split into three different groups: a) Fresh Group (Control): secondary follicles were isolated without any previous vitrification; b) Follicle-Vitrification group (Follicle-Vit): secondary follicles were vitrified in isolated form c) Tissue-Vitrification group (Tissue-Vit): secondary follicles (formed in situ) were vitrified within fragments of ovarian tissue, and isolated subsequently after warming. From all groups, isolated secondary follicles were in vitro cultured for 18 days. During in vitro culture, the levels of hormones secretion (progesterone and estradiol), antrum formation and growth rate were measured. After in vitro culture the cumulus-oocyte complexes (COCs) were recovered from cultured follicles and submitted to in vitro maturation (IVM). In addition, the viability and chromatin configuration of the oocytes were also evaluated Ovarian cortex fragmentation and follicle isolation The ovarian cortex was sectioned into pieces of 1 to 2 mm thickness under sterile conditions in a Petri-dish containing MEM HEPES supplemented with antibiotics (100 µg/ml penicillin and 100 µg/ml streptomycin). Fragments obtained were transferred to another Petri-dish containing fresh medium and then visualized under a stereomicroscope (Nikon SMZ 645, Tokyo, Japan; 100 x). Once localized, secondary follicles, with average diameter of µm (range µm), were mechanically isolated by microdissection using a 26-G needle attached to 1 ml syringe. Only
101 79 those follicles with the oocyte-follicle complex visible, surrounded by several layers of granulosa cells with intact basement membrane and no antral cavities were selected for this study In vitro culture of sheep secondary follicles Secondary follicles were transferred into drops of 100 µl of culture medium under mineral oil in Petri-dishes (60 x 15 mm) and cultured for 18 days (one follicle/drop) at 39 C and 5% CO2 in air. The culture medium used was composed MEM alpha (α-mem) supplemented with 10 µg/ml insulin, 5.5 µg/ml transferrin, 5 ng/ml selenium, 2 mm glutamine, 2 mm hypoxanthine, 3 mg/ml bovine serum albumin (BSA), 50 µg/ml ascorbic acid, 50 ng/ml leukemia inhibitory factor (LIF) and 50 ng/ml Kit Ligand (KL). This medium was chosen based on our previously study [15] Morphological analysis and assessment of in vitro follicular growth At day 0, 6, 12 and after 18 days of in vitro culture, the percentage of morphologically normal follicles and follicular diameters from Control, Follicle-Vit and Tissue-Vit were determined. Follicles were classified as morphologically normal if they presented an intact basement membrane, no extrusion of the COCs from the follicle, bright and homogeneous granulosa and theca cells. Follicle degeneration was recognized when the oocytes and surrounding cells were darkened, had misshaped oocytes or decreased follicle diameter. Antral cavity formation was defined as a visible translucent cavity within the granulosa cell layers. Follicular diameter was measured at the basement membrane (from the major and minor axes) of each follicle with the aid of an ocular micrometer inserted into a stereomicroscope (SMZ 645 Nikon, Tokyo, Japan; 100 x) on days 0, 6, 12 and 18 of in vitro culture. The average of these 2 measurements was used to determine the follicle diameter only in morphologically normal follicles. The daily mean increase in follicular diameter (daily follicular growth rate) was calculated as the diameter of morphologically normal follicles at day 18 minus the diameter of the same follicle at day 0, divided by the total number of days in culture Vitrification protocol used to ovarian isolated follicles and fragments Ovarian fragments (1 to 2 mm thick) and isolated secondary follicles were exposed to different concentrations (12.5, 25, 50 and 100%) of vitrification solution composed by MEM HEPES supplemented with 10% fetal bovine serum (FBS), 2.60 M acetamide, 2.62 M dimethylsulfoxide, 1.31 M 1, 2-propanediol and M polyethylene glycol. Samples were exposed to the first two concentrations (12.5 and 25%) of vitrification solution for 5 minutes at
102 80 room temperature; then to the next two concentrations (50 and 100%) of vitrification solution for 15 minutes (for fragments) or 5 minutes (for isolated follicles) at 4 C. Following, samples (either ovarian fragments or isolated follicles) were placed on the surface of a metal cube partially submerged in liquid nitrogen and transferred to cryovials (10 follicles or 10 fragments each) in accordance with protocol adapted from Lunardi et al. [15]. Samples were kept into liquid nitrogen (196 C) for 6 days Warming protocol of ovarian follicles and fragments Cryovials containing samples of ovarian tissue and isolated secondary follicles were removed from liquid nitrogen and exposed to room temperature for 1 minute and immersed in a water bath (37 ºC) for 30 seconds. Soon after, removal of cryoprotectants was performed by immersion of ovarian fragments or secondary follicles in washing solutions, three baths of 5 minutes each with the solution composed out of MEM HEPES plus 10% FBS and decreasing concentrations of sucrose (0.5, 0.25 and 0.0 M) in accordance with protocol adapted from Lunardi et al. [16] Steroid production To evaluate 17β-estradiol (pmol/l) and progesterone (nmol/l) concentrations during in vitro culture, the total of 30 pooled samples of conditioned medium from 60 growing follicles (2 follicles per medium, 10 samples per group) follicles from Control and each of the vitrification groups at days 6, 12 and 18 were retrieved and stored at -80 C. The analysis of 17β-estradiol (pmol/l) and progesterone (nmol/l) were measured by chemiluminescence using an immunoassay system (VitrosEci/EciQ Immunodiagnostic System, Johnson & Johnson Company, Ortho-Clinical Diagnostics, Buckinghamshire, United Kingdom) according to manufacturer s instructions In vitro maturation (IVM) of COCs At the end of the culture period, the COCs obtained from in vitro cultured were carefully harvested from intact follicles using 26-G needles and from extrude follicles under a stereomicroscope. From In Vivo Grown Control, in vivo-grown COCs were collected from antral ovarian follicles. Oocytes surrounded by at least one compact layer of cumulus cells were selected for IVM. The recovery rate was calculated by dividing the number of COCs by the number of viable follicles at day 18 of culture multiplied by 100. The selected COCs were washed in medium composed of tissue culture medium HEPES (TCM199H) supplement with pyruvate (0.911
103 81 mm/l) and 10% FBS followed by in vitro maturation medium. The maturation medium was composed by tissue culture medium sodium bicarbonate (TCM199B) supplemented with 0.5 µg/ml recombinant bovine follicle-stimulating hormone (rbfsh) (NANOCORE, Campinas, São Paulo, Brazil), 5 µg/ml (luteinizing hormone) LH, 1 µg/ml 17β-estradiol, 10 ng/ml recombinant epidermal growth factor (EGF), mm/l pyruvate, 100 µm/l cysteamine, 50 ng/ml recombinant insulin-like growth factor I (IGF-I) and 1% BSA. After washing, COCs were transferred to 50 µl drops of maturation medium under mineral oil and then incubated for 36 to 40 hours at 39 oc with5% CO2 according to Luz et al. [17] Assessment of oocyte viability and chromatin configuration After IVM, the COCs were denuded from surrounding expanded cumulus cells by manual pipetting in TCM199 HEPES containing 0.1% hyaluronidase and subjected to viability analysis. To this end, the oocytes were incubated in 100 µl drops of 2 µm ethidium homodimer-1 supplemented with 4 µm calcein-am, 0.5% glutaraldehyde and 10 µm Hoechst at room temperature for 30 min. After this, the oocytes were washed in TCM199 HEPES and were visualized under fluorescence microscopy (Nikon Eclipse 80i, Tokyo, Japan; 40 x). Oocytes were considered viable if the cytoplasm was positively stained with calcein-am (green) and not stained with ethidium homodimer-1 (red). The emitted fluorescent signals of calcein-am and ethidium homodimer - 1 were collected at 488 nm and 568 nm, respectively. In addition, oocytes were stained with Hoechst (Molecular Probes, Invitrogen, Karlsruhe, Germany) and then analyzed for chromatin configuration being emitted fluorescent signals at 483 nm. This dye was employed to analyze the oocyte s chromatin configuration through observation of the intact germinal vesicle (GV), meiotic resumption (including germinal vesicle breakdown - GVBD, metaphase I - MI, anaphase I - AI or telophase I - TI) or nuclear maturation (metaphase II - MII) Statistical analysis Data for discrete variables (morphologically normal follicles, antrum formation, recovery rate and chromatin configuration of oocytes) were analyzed as dispersion of frequency using Chisquare test. Otherwise, when the observed frequency was equal or less than five units, Exact Fisher s Test was applied. In both cases, results were expressed as percentages. Data for continuous variables (follicular diameter, growth rate after culture and hormonal dosages) Kruskall Wallis and Unpaired Test-t were initially evaluated for homocedasticity and normal distribution of the residues, by Bartlett s and Shapiro-Wilk tests, respectively. Confirmed both requirements underlying analysis of variance, the effects of treatment, time of culture and treatment by time
104 82 interaction were analyzed using PROC MIXED of SAS (2002), including repeated statement to account for autocorrelation between sequential measurements. The model was Yijk=µ+Ri+Fj+Tk+(RT)ik+eijk, where Yijk is the observation of the jth follicle in the ith treatment at the kth time of culture, µ is the overall mean, Ri is the ith treatment, Fj is the random effect of the jth follicle within the ith treatment, Tk is the kth time of culture, (RT)ik is the treatment by time interaction term and eijk is the random residual effect. Comparisons among treatments or times were further analyzed by the Student-Newman-Keuls (SNK) test. A probability of P<0.05 indicated a significant difference and results were expressed as mean ± standard deviation (DP) (oocyte diameter) or expressed as mean ± standard error of the mean (SEM) (follicle diameter, growth rate). 3. Results 3.1. Assessment of morphologically normal follicles and antral cavity formation during culture A total of 154 secondary follicles were distributed randomly to groups Control (n = 50), Follicle-Vit (n = 40) and Tissue-Vit (n = 64), and in vitro cultured for 18 days. The percentage of morphologically normal follicles was similar between Follicle-Vit (97.5%, n = 39/40) and TissueVit (93.75%, n = 60/64) being both higher (P< 0.05) than in Control group (78%, n = 39/50) (Figure 1). At the end of culture period, some follicles showed rupture of the basal membrane and, consequently, released their COCs. In Control (20%, n =10/50), there was an increase (P < 0.05) in the percentage of extruded oocytes when compared to Tissue-Vit (1.56%, n =1/64). Interestingly, the Follicle-Vit (n = 0/40) did not present follicle extrusion. The percentage of degenerated follicles was similar (P > 0.05) among the three groups (Control: 2%; Follicle-Vit: 2.5% and Tissue-Vit: 4.69%). Except for Follicle-Vit, the percentage of antrum formation increased significantly from day 6 to day 18 of culture. A significantly higher percentage of antrum formation was observed in the Tissue-Vit compared to the Control (Figure 2) Follicle diameter and daily growth rate before and after vitrification and in vitro culture of ovine preantral follicles Follicle diameter increased significantly from day 0 to day 18 of culture only in the Control and Follicle-Vit groups. However, no differences were observed among the groups regardless the culture time (P>0.05) (Figure 3).
105 83 The Figure 4a illustrates the follicular daily growth rate in the three groups throughout the period of in vitro culture. The Control (7.05 ± 1.07 µm/day, n = 21) and Follicle-Vit (5.02 ± 0.63 µm/day, n = 38) were similar between themselves, and higher (P<0.05) than Tissue-Vit (2.42 ± 0.32 µm/day, n = 44). The Figure 4b shows the percentage of follicles with decreased diameter throughout the in vitro culture. The percentage of follicles with decreased diameter in Follicle-Vit (2.56%, n = 1/39) was lower (P < 0.05) than Control (46.15% n = 18/39) and in Tissue-Vit (26.67%, n =16/60). 3.3 Steroid production The follicles from Control produced higher (P < 0.01) estradiol levels than vitrified groups. Besides, only in the Control estradiol production increased significantly from day 6 to day 18 of culture, only in Follicle-Vit progesterone levels increases significantly from day 6 to day 18 (Table 1) Oocyte viability and diameter, recovery rate of oocytes cultured in vitro and meiotic stages of sheep oocytes from preantral follicles after long-term culture The recovery rate of oocytes from normal intact cultured follicles was similar between vitrified and Control. However, the Follicle-Vit had a higher (P < 0.05) percentage of recovered oocytes when compared to Tissue-Vit. Recovered oocytes from antral follicles that grown in vivo (In Vivo Grown) had larger (P < 0.05) diameter than oocytes cultured in vitro. Moreover, oocytes from Control (fresh cultured secondary follicles) had higher (P < 0.05) diameter when compared to oocytes from Follicle-Vit and Tissue-Vit groups. The vitrification procedure of secondary follicles (isolated or enclosed on ovarian stroma), did not affected oocyte size after in vitro maturation (Table 2). After in vitro maturation, all oocytes were incubated with fluorescent labels calcein-am, ethidium homodimer-1 and Hoechst to evaluate viability and chromatin configuration, respectively. Tissue-Vit Group had higher (P < 0.05) oocyte degeneration rate (33.33%) when compared to other groups (In Vivo Grown: 6.66%; Control: 8.69% and Follicle-Vit: 7.69%). When evaluating chromatin configuration, the percentage of meiotic resumption (GVBD + MI + MII) in Control, Follicle-Vit and Tissue-Vit did not differ, but they were lower (P<0.05) than In Vivo Grown. In vitrified groups, only Follicle-Vit had one oocyte in MI. As expected, In Vivo Grown oocytes showed a significantly higher percentage of MII oocyte (Table 2) than in Control (Figure 5).
106 84 4. Discussion In the present study, the follicular development, as well as the oocyte in vitro maturation were evaluated after two different forms of vitrification of sheep secondary follicles: vitrification in isolated form (without much stroma) or vitrification in in situ form (within ovarian tissue fragments). Therefore, secondary follicles were first isolated from ovarian fragments and then vitrified, ie, the Follicle-Vitrification group (Follicle-Vit) or fragments of ovarian tissue that were first vitrified, followed by isolation of the secondary follicles, composing the Tissue-Vitrification group (Tissue-Vit). Our data showed that the follicles from Follicle-Vit had higher follicular growth rate, oocyte viability and recovery rate. Similar results were reported by Hatami et al. [4] in mice, were the vitrification of isolated preantral follicles was better than vitrification of the whole ovary. These authors observed better follicular survival, antrum formation and oocyte maturation rates. These results may be due to different conditions under which follicles are submitted when vitrified, within or outside of the ovarian stroma. Follicles and oocytes vitrified included in ovarian tissue may not be fully penetrated by intracellular cryoprotectants, due to the large number of cells that surround them. Moreover, the differences among the various cell types present in the ovarian tissue can interfere with the permeability of cryoprotectants, leading follicles to an increased susceptibility to injuries, during cooling. The ovarian fragments were ~2 mm thickness, while their secondary follicle has an average diameter of µm, thereby, the spread of the cryoprotectant into the tissue, during the period of exposure to vitrification solutions, may not have occurred homogeneously, resulting in an irregular dehydration leaving a substrate for ice formation [14, 18]. A previous study showed a high percentage of follicles with decreasing diameter in culture of 6 days when they had previously undergone vitrification [15]. In the present study, we showed that when the follicles were vitrified in isolated form (Follicle-Vit) only 2.56% had a diameter reduction which was lower than the reduction of follicles vitrified in the tissue (Tissue-Vit) or fresh follicles (Control). Reduction in follicular diameter may occur due to dehydration and osmotic variations suffered during vitrification [19]. This is an acute phenomenon and their effect must be temporary. Follicular diameter should be restored after a few hours of in vitro culture. Studies indicate that if the diameter is not reestablished (as we observed in this study even after 18 days of in vitro culture) it can be considered an indication of follicular degeneration [6, 7, 20]. In the present study, the Follicle-Vit (5.02 ± 0.63 µm; ± pmol/l) treatment had follicular growth rate similar to the control group. However, the estradiol production on the same treatment was lower than control (7.05 ± 1.07 µm; 4,729.0 ± 1,200.0 pmol/l). The variation in
107 85 estradiol production may be due to spontaneously luteinization of these follicles cells and not characterize the proliferative or follicular phase of normal estrous cycle. Although, luteinized cells are not typical of follicles that will be recruited and will gain dominance in the normal ovarian folliculogenesis. Xing et al. [21] demonstrated a lower estradiol production and a greater diameter in rats isolated secondary follicles after vitrification. Otherwise, during vitrification process the cells can decrease cell metabolism and, consequently decrease the estradiol production in an attempt to compensate the potential damage. Moreover, Silva et al. [22] and Cecconi et al. [23] previously reported that estradiol synthesis and follicular growth pattern are not strictly dependent on each other. Both treatments with vitrified follicles in our experiment had lower production of estradiol than the Control, probably because the internal theca cells, and even part of granulosa cells have been possibly damaged by the toxic effect of cryoprotectants. The internal theca cells secrete androstenedione, an androgenic precursor that is transferred through the basal lamina to the granulosa cells culminating in the production of testosterone, which is then converted to estradiol by aromatase [24]. These cells damage during vitrification procedures compromises the intrinsic machinery of hormone production, and certainly the oocyte growth and competence for development will also be damaged. Therefore, it becomes necessary deeper studies of methods that preserve the functionality of theca and granulosa cells after vitrification to ensure and to maintain adequate production of estradiol to support the long term culture and better maturation rate. In this study, the oocytes from follicles previously vitrified showed significantly smaller diameter, after in vitro maturation, than the oocytes from follicles in vitro cultured without prior vitrification. In a prior study, it has been shown that most oocytes vitrified in isolated secondary follicles cease their growth in the first six days of culture and vitrified oocytes in secondary follicles enclosed in ovarian tissue, not only stop growing, in the same period of in vitro culture, as well as reduce in diameter [15]. In the Tissue-Vit group the cooling of the oocyte is slow and gradual, as occurs in peripheral region to the interior of the follicle, where is situated the oocyte [4]. In Follicle-Vit group, the oocyte is free of barriers such as the ovarian stroma, so the cooling is faster. As a high cooling rate is associated with the absence of ice crystal formation [18], we suggest that this might be the reason why the oocytes from Follicle-Vit had a higher post-thaw viability than oocytes from Tissue-Vit group. Other studies [25, 26] have also demonstrated that the final oocyte diameter from in vitro cultured follicles remains smaller than the diameter of oocytes from In Vivo Grown follicles. This may be an early sign of degeneration, possibly due to injuries in the connecting structures between oocyte and surrounding granulosa cells, since it is known that the vitrification pronouncedly reduces the oocyte microvilli [15]. The loss of these microvilli may impair the oocyte-somatic cells communication and reduces their growth characteristics [21].
108 86 This study showed for the first time, that sheep oocytes from vitrified secondary follicles (Follicle-Vit) cultured in vitro, for 18 days, are able to resume meiosis (germinal vesicle breaks), and also reach the stage of MI, after in vitro maturation, although with lower rate compared to In Vivo Grown oocytes. Although many efforts and promising results have been reported by different teams regarding the in vitro development of preantral follicles in different species [10], we believe that the conditions of in vitro culture of these follicles, which ensure the full oocyte growth, need to be improved, especially to oocytes previously submitted to cryopreservation. We also believe that the determination of an ideal system for the in vitro culture of preantral follicles cryopreserved or not, is absolutely necessary, whereas the transplantation of ovarian tissue offers risks of reintroduction of cancer cells to patients in remission. Considering that isolated follicles display a better follicular growth rate, oocyte viability and fewer follicles with decreased diameter in vitro culture, after vitrification, we conclude that with our current method of vitrification the strategy of vitrification after isolation of secondary follicles provided a better substratum for growing the enclosed oocytes. 5. Acknowledgements: This work was supported by CNPq. Franciele Osmarini Lunardi is a recipient of a grant from CAPES Brazil. In addition, Ana Paula Ribeiro Rodrigues and José Ricardo de Figueiredo are recipients of a grant from CNPq Brazil. Johan Smitz is Especial Visitor Researcher from CAPES.
109 87 Figures Figure 1. Percentage of isolated morphologically normal sheep preantral follicles without vitrification (Control, n = 50) and after vitrification of isolated follicles (Follicle-Vit, n = 40) or ovarian tissue (Tissue-Vit, n = 64) at days 0, 6, 12, and 18 of in vitro culture. AB Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day. a,b Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group. Figure 2. Normal sheep secondary follicle before (Day 0) in vitro culture (a) or cultured for 6 days (b), 12 days (c) or 18 days (d) in Follicle-Vit group. Note beginning of antrum formation at day 6 of in vitro culture. (e) Percentage of antrum formation of sheep preantral follicles without
110 88 vitrification (Control, n = 39) and after vitrification of isolated follicles (Follicle-Vit, n = 39) or ovarian tissue (Tissue-Vit, n = 60) at days 6, 12, and 18 of in vitro culture. A,B Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day. a,b Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group. Figure 3. Follicular diameter (µm) (mean ± SEM) of sheep preantral follicles without vitrification (Control, n = 21) and after vitrification of isolated follicles (Follicle-Vit, n = 38) or ovarian tissue (Tissue-Vit, n = 44) at days 0, 6, 12, and 18 of in vitro culture.a Different uppercase letters indicate statistically significant differences among groups (P<0.05) within the same day.a,b,c Different lowercase letters indicate statistically significant differences among days of culture (P<0.05) within the same group.
111 89 Figure 4. (a) Daily growth rate (µm/day) (mean ± SEM) of sheep preantral follicles without vitrification (Control, n = 21) and after vitrification of isolated follicles (Follicle-Vit, n = 38) or ovarian tissue (Tissue-Vit, n = 44) after 18 days of in vitro culture and (b) Percentage of sheep preantral follicles with decreased diameter from Control (n = 18), Follicle-Vit (n = 1) e Tissue-Vit (n = 16) after 18 days of culture. A,B,C Different uppercase letters indicate statistically significant differences among groups (P<0.05). Figure 5. Viable oocyte in metaphase I after IVM from Follicle-Vit group after in vitro culture for 18 days. Note: Brightfield (a), calcein-am (b) and Hoechst (c).
112 90 Table 1. Oocyte viability (%) and diameter (µm ± SD), recovery rate of oocytes cultured in vitro (%), and meiotic stages (%) of sheep oocytes from preantral follicles after long-term culture (18 days) in Control, Follicle-Vit and Tissue-Vit groups. No of oocytes o N of oocytes Groups recovered / No normal follicles (%) o N of viable o oocytes / N of oocytes (%) Mean oocyte diameter (µm) No of GVBD o with meiosis resumption / N oocytes / N of o of viable oocytes oocytes meiosis resumption (%) (%) No MI oocytes / No MII oocytes / No of oocytes No of oocytes meiosis meiosis resumption (%) resumption (%) In Vivo Grown - 28/30 (93.3) A ± A 26/28 (92.85) A 9/26 (34.61) B 1/26 (3.84) B 16/26 (61.53) A Control 23/39 (58.97) AB 21/23 (91.3) A ± 9.18 B 8/21 (38.09) B 4/8 (50.00) AB 3/8 (37.50) A 1/8 (12.50) B Follicle-Vit 26/39 (66.67) A 24/26 (92.3) A ± C 11/24 (45.83) B 10/11 (90.90) A 1/11 (9.09) AB 0/11 (0.0) Tissue-Vit 24/60 (40.00) B 16/24 (66.7) B ± C 6/16 (37.5) B 6/6 (100) A 0/6 (0.0) 0/6 (0.0) ABC Different uppercase letters indicate statistically significant differences (P<0.05) among groups (column).
113 91 Table 2. 17β-estradiol (pmol/l) (mean ± SEM) and progesterone (nmol/l) concentration (mean ± SEM) in pooled media collected on the different days during long-term culture of preantral follicles in Control (n = 20), Follicle-Vit (n = 20) and Tissue-Vit (n = 20) groups. Groups Estradiol Progesterone Day 6 Day 12 Day 18 Day 6 Day 12 Day 18 1,989.0 ± 1,270.0 ba 2,792.0 ± abA 4,729.0 ± 1,200.0aA ±3aB 7.0 ± 1.2aC Follicle-Vit ± 31.0aB ± 24.0bC ± 100.0aB 9.0 ± 6bB 12.0 ± 3bA 25.0 ± 7aA Tissue-Vit ± 38.0aB ± 61.0aB ± 180.0aB 16 ± 6.0aB 13.0 ± 2.0bA 15.0 ± 1.0aB Control a-b Within a row, values without a common superscript differed among days (P<0.01) A,B,C Within a column, values without a common superscript differed among groups (P<0.01).
114 92 6. References 1. Shaw JM, Bowles J, Koopman P, Wood EC, Trounson AO. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Human Reproduction 1996; 11: Dolmans M-M, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010; 116: Rosendahl M, Andersen MT, Ralfkiaer E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertility and Sterility 2010; 94: Hatami S, Zavareh S, Salehnia M, Lashkarbolouki T, Ghorbanian MT, Karimi I. The impact of alpha lipoic acid on developmental competence of mouse vitrified pre-antral follicles in comparison to those isolated from vitrified ovaries. Iran J Reprod Med 2014, 12: O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biology of Reproduction 2003; 68: Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010; 140: Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Human Reproduction 2011; 26: Saraiva MVA, Rossetto R, Brito IR, Celestino JJH, Silva CMG, Faustino LR, Almeida AP, Bruno JB, Magalhaes DM, Matos MHT, Campello CC, Figueiredo JR. Dynamic Medium Produces Caprine Embryo From Preantral Follicles Grown In vitro. Reproductive Sciences 2010; 17: Arunakumari G, Shanmugasundaram N, Rao VH. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology 2010; 74: Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertility and Sterility 2013; 99: Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertility and Sterility 2006; 86: Wang XQ, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction 2011; 141:
115 Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, Tucker MJ. Potential importance of vitrification in reproductive medicine. Biology of Reproduction 2002; 67: Zhou XM, Qiao WT, Zhang XL, Liu Z, Gao DY. Physical modeling of flow boiling in microchannels and its induced vitrification of biomaterials. International Journal of Heat and Mass Transfer 2015; 83: Lunardi FO, Chaves RN, de Lima LF, Araújo VR, Brito IR, Souza CE, Donato MA, Peixoto CA, Dinnyes A, Campello CC, de Figueiredo JR, Rodrigues AP. Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture. Cell and tissue research 2015, Epub ahead of print. 16. Lunardi FO, Araujo VR, Faustino LR, Carvalho AdA, Braga Goncalves RF, Bass CS, Bao SN, Olazia Name KP, Campello CC, de Figueiredo JR, Ribeiro Rodrigues AP. Morphologic, viability and ultrastructural analysis of vitrified sheep preantral follicles enclosed in ovarian tissue. Small Ruminant Research 2012; 107: Luz VB, Araujo VR, Duarte ABG, Celestino JJH, Silva TFP, Magalhaes-Padilha DM, Chaves RN, Brito IR, Almeida AP, Campello CC, Feltrin C, Bertolini M, et al. Eight-Cell Parthenotes Originated From In vitro Grown Sheep Preantral Follicles. Reproductive Sciences 2012; 19: Devismita D, Kumar A. Effect of cryoprotectant on optimal cooling rate during cryopreservation. Cryobiology 2015; 70: Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: Structural normality of follicles after thawing and culture in vitro. Cryobiology 1999; 38: Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Human Reproduction 2011; 26: Xing W, Zhou C, Bian J, Montag M, Xu Y, Li Y, Li T. Solid-surface vitrification is an appropriate and convenient method for cryopreservation of isolated rat follicles. Reproductive Biology and Endocrinology 2010; Silva CMG, Castro SV, Faustino LR, Rodrigues GQ, Brito IR, Rossetto R, et al. The effects of epidermal growth factor (EGF) on the in vitro development of isolated goat secondary follicles and the relative mrna expression of EGF, EGF-R, FSH-R and P450 aromatase in cultured follicles. Research in Veterinary Science. 2013;94: Cecconi S, Barboni B, Coccia M, Mattioli M. In vitro development of sheep preantral follicles. Biology of Reproduction 1999; Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149:R193-R Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O(2) and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Human Reproduction 2001; 16:
116 Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertility and Sterility 2010; 93:
117 95 9 CAPÍTULO 4 Folículos ovinos secundários isolados e vitrificados são capazes de produzir oócitos em metáfase II após cultivo e maturação in vitro Sheep isolated secondary follicles are able to produce metaphase II oocytes after vitrification and long-term in vitro culture Periódico: Biology of Reproduction, (Em fase de correção) (ISSN ), Qualis A1
118 96 RESUMO A vitrificação de folículos pré-antrais seguida do cultivo in vitro (CIV) visa a obtenção de oócitos potencialmente fertilizáveis. Contudo, um estudo anterior mostrou que a taxa de retomada da meiose de oócitos vitrificados oriundos de folículos secundários (isolados ou inclusos no estroma ovariano) ainda é muito baixa e, pode ser devido à ineficiência dos meios de cultivo utilizados. Portanto, visando melhorar o desenvolvimento folicular, bem como as taxas de retomada da meiose oocitária, o objetivo deste estudo foi comparar a eficiência do α-mem e do TCM199 sobre o desenvolvimento in vitro de folículos secundários previamente vitrificados (isolados ou inclusos no estroma ovariano ovino). Folículos secundários isolados frescos ou vitrificados foram submetidos ao cultivo in vitro por 18 dias nos meios α-mem ou TCM 199, resultando em seis tratamentos: controle α-mem, Controle TCM 199, Folículo-Vit α-mem, Folículo-Vit TCM199, Tissue-Vit αmem e Tissue-Vit TCM199. Após o cultivo in vitro, os complexos cumulos-oócitos (CCO s) de todos os tratamentos, bem como de folículos crescidos in vivo foram recuperados e submetidos à matruação in vitro (MIV), enquanto as células da granulosa, provenientes dos folículos cultivados, foram destinadas para avaliação da expressão gênica (CX37, CX43, BAX e BCL2). Os dados foram submetidos à ANOVA, e as médias foram comparadas pelo teste Student-Newman-Keuls (diâmetro folicular e taxa de crescimento após cultivo), e por Qui-quadrado (folículos morfologicamente normais, formação de antro, taxa de recuperação e configuração da cromatina oocitária). Diferenças foram significativas quando P < 0,05. O percentual de formação de antro foi maior nos grupos Follicle-Vit do que nos Tissue-Vit em ambos meios após CIV. Após IVM, o diâmetro oocitário não foi afetado pela vitrificação ou cultivo folicular em ambos meios. Após IVM oócitos provenientes do Follicle-Vit α-mem alcançaram o estágio de metáfase (MII). A vitrificação reduziu a expressão dos genes CX37, CX43 e BAX, e aumentou o BCL2. Conclui-se que o número de oócitos recuperados após cultivo in vitro, bem como o diâmetro oocitário após a maturação in vitro não foram influenciados pela forma de vitrificação ou meio de base utilizado para o cultivo folicular. Após a vitrificação, apenas oócitos oriundos de folículos, cultivados em α-mem atingiram estágio de MII. Palavras - chave: Follicular development. Connexins. α-mem. TCM199. Metafase MII
119 97 Sheep isolated secondary follicles are able to produce metaphase II oocytes after vitrification and long-term in vitro culture Franciele Osmarini Lunardi1, Francisco Leo Nascimento de Aguiar1, Livia Brunetti Apolloni1, Ana Beatriz Graça Duarte2, Naiza Arcângela Ribeiro de Sá1, Érica Suzanne Soares Leal1, Antonia Debora Sales1, Carlos Lobo1, Johan Smitz5, Cláudio Cabral Campello1, José Ricardo de Figueiredo1, Ana Paula Ribeiro Rodrigues1. 1 Laboratory of Manipulation of Oocytes and Ovarian Pre-antral Follicles (LAMOFOPA), Faculty of Veterinary of Ceará State University, Fortaleza, Ceará, Brazil. 2 Universidade da Integração Internacional da Lusofonia Afro-Brasileira (UNILAB). 3 Ceará State University, Fortaleza, Ceará, Brazil. 4 Center of Experimental Biology (Nubex), University of Fortaleza (UNIFOR), Fortaleza, Ceará, Brazil 5 Follicle Biology Laboratory, Center for Reproductive Medicine, UZ Brussel, Laarbeeklaan 101, B-1090 Brussels, Belgium. Abstract The vitrification of preantral follicles followed by in vitro culture (IVC) is a viable tool to produce fertilizable oocytes. However, the meiotic resumption rates of oocytes from vitrified secondary follicles (SF) remains low (either enclosed in the ovarian stroma or in isolated form). Therefore, this study aimed to verify whether vitrified SF need different culture media to complete its development, testing two base media (α-mem and TCM199) during IVC for 18 days. Sheep ovarian fragments were divided in six groups: 1) Fresh group (Control α-mem and Control TCM199): SF without previous vitrification; 2) Follicle-vitrified (Follicle-Vit α-mem and FollicleVit TCM199): SF vitrified after isolation; and 3) Tissue-Vitrified (issue-vit α-mem and Tissue-Vit TCM199): SF vitrified enclosed in ovarian fragments (in situ form) and subsequently isolated. The isolated SF were submitted to IVC for 18 days in α-mem or TCM199 media. After IVC, the recovered cumulus-oocyte complexes (CCO's) underwent in vitro maturation (IVM) followed by the evaluation of chromatin configuration. In addition, follicular granulosa cells were undergone to gene expression of BAX, BCL2, Connexins 37 and 43 (CX37 and 43). Some CCO s from in vivo developed antral follicles were used as in vivo control. Data were analyzed by ANOVA, and media were compared by Student-Newman-Keuls and Qui-square tests. Differences were considered significant when (P < 0.05). Follicle-Vit group had higher (P < 0.05) percentage of antrum formation when compared to Tissue-Vit in both media after IVC. After IVM, vitrification does not
120 98 affected (P > 0.05) oocyte diameter of the groups. Oocytes from Follicle-Vit in α-mem reached metaphase II stage after IVM. The gene expression for CX37, CX43 and BAX, was lower on vitrified Tissue-Vit groups (α-mem or TCM199). In contrast the BCL2 expression was higher on these groups. In conclusion, the vitrification of ovine SF after previous isolation favors in vitro follicular development and oocyte maturation after IVC in α-mem medium. Keywords: Metaphase II, Connexins, BAX, BCL2, α-mem, TCM Introduction The cryopreservation of preantral follicles enclosed on ovarian cortex represents an important tool for the preservation of reproductive potential on young women facing cancer treatment (Donnez; Dolmans et al., 2014). After the cancer remission, these women can receive the cryopreserved ovarian graft and restore their reproductive and endocrine competence (Donnez; Dolmans et al., 2011). However, in some types of cancer as leukemia, neuroblastoma or metastasis the transplantation of ovarian tissue could reintroduce cancerous cells to the patient (Donnez; Dolmans et al., 2015). Optionally, the in vitro culture of isolated preantral follicles, followed by oocyte maturation represents a good and safe alternative to avoid the reintroduction of cancer cells. Finally, these oocytes could be fertilized and the derived embryos that would be transferred to realize the motherhood dream of cancer survivors. A previous study reported the in vitro production of murine embryos (Eppig et al. 2003) from preantral follicles (primordial) cultured in vitro. Later, the embryo production from developing preantral follicles was also showed on goats (Magalhães et al., 2010) and sheep (Arunakumari et al., 2010). Nonetheless, these results were obtained with fresh preantral follicles what can be unfeasible in some cases. Rodent embryos were produced in vitro from vitrified and cultured secondary follicles (Wang et al., 2011). To our knowledge there are no reports of embryo production from cryopreserved follicles in domestic species. Recently we demonstrated that sheep ovarian follicles isolated from ovarian cortex, vitrified and cultured are able to resume meiosis after in vitro maturation. However, only 9.09% of these oocytes achieved the metaphase I stage, which is considered very low (Lunardi et al., 2015). Based on our own results and other reports (Castro et al., 2014), we believe that cryopreserved follicles have different requirements for in vitro growth and development when compared to fresh follicles. Furthermore, cryopreserved follicles are more susceptible to atresia process during the in vitro culture Men et al. (2003). Thus, grounded on these observations, we
121 99 hypothesized that follicular development after vitrification could be influenced by the media composition during the in vitro culture. Therefore, in order to improve the follicular development and meiosis resumption rates, the objective of this study was to compare the efficiency of different culture media (α-mem and TCM199) on the in vitro culture of secondary follicles that were previously vitrified enclosed on ovarian tissue or isolated. Thus, several parameters were evaluated as follicular growth, survival and antrum formation after 18 days of in vitro culture. The oocyte viability and maturation rates were evaluated as well. Furthermore, the integrity of the connections involved with the exchange of substances between the cumulus cells and oocyte was evaluated by gene expression of connexins 37 and 43. The expression of the genes associated with apoptosis or atresia (BAX and BCL2) was evaluated on granulosa cells after the in vitro culture. 2. Materials and methods 2.1. Source of ovaries Ovaries (n = 90) were collected at abattoirs from 45 adult mixed-breed ewes. Immediately post-mortem, under aseptic conditions, the ovaries were washed in 70% alcohol for 10 seconds, followed by two washes in HEPES buffered minimum essential medium (MEM) supplemented with 100 µg/ml penicillin and 100 µg/ml streptomycin. Each pair of ovaries was transported to the laboratory in tubes containing 15 ml of MEM at 4 C within 4 h. The chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise mentioned Experimental design According our previous study description (Lunardi et al., 2015), at the laboratory, ovaries were stripped out from surrounding fat and fibrous tissue, and the cortex portions were recovered and fragmented in small pieces (1 to 2 mm thick) and split in three different conditions: a) Non Vitrified fragments: fresh secondary follicles were isolated (Control); b) Follicle-Vitrification (Follicle-Vit): secondary follicles were isolated and then vitrified and c) Tissue-Vitrification (Tissue-Vit): secondary follicles were vitrified enclosed on ovarian fragments (in situ form), and isolated subsequently after warming. From all three conditions the isolated secondary follicles were submitted to in vitro culture for 18 days in two different culture base media: MEM alpha modification (α-mem) or Tissue Culture Medium 199 (TCM199) corresponding to six treatments: Control α-mem, Control TCM199, Follicle-Vit α-mem, Follicle-Vit TCM199, Tissue-Vit α-mem and Tissue-Vit TCM199 (Figure 1).
122 100 The percentage of morphologically normal follicles, antral cavity formation, follicle diameter and daily growth rate were measured during the in vitro culture. After culture period the cumulus-oocyte complexes (COCs) were recovered from cultured follicles and submitted to in vitro maturation (IVM). The viability and chromatin configuration of the matured oocytes were also evaluated. Furthermore, granulosa cells were randomly assigned to Real Time PCR (qpcr) analysis to evaluate the relative expression of connexins 37, 43, BAX and BCL2 after 18 days of in vitro culture from Fresh, Follicle-Vit and Tissue-Vit groups in both base media (α-mem or TCM199) Ovarian cortex fragmentation and follicle isolation. The ovarian cortex was sectioned in small pieces of 1 to 2 mm thickness under sterile conditions in a Petri-dish containing α-mem supplemented with HEPES and antibiotics (100 µg/ml penicillin and 100 µg/ml streptomycin). The ovarian fragments were transferred to another Petri-dish containing fresh medium and then visualized under a stereomicroscope (100x, Nikon SMZ 645, Tokyo, Japan). Once located, secondary follicles, with average diameter of 340 µm (range µm), were mechanically isolated by microdissection with the aid of 26G needle attached to 1 ml syringe. Only follicles with a visible oocyte-follicle complex, surrounded by several layers of granulosa cells with intact basement membrane and no antral cavities were selected for this study Vitrification procedure The vitrification solution previously defined by Bordes et al. (2005) was used with some amendments as: MEM supplemented with 10% fetal bovine serum (FBS), 2.60 M acetamide, 2.62 M dimethylsulfoxide, 1.31 M 1, 2-propanediol (PROH) and M polyethylene glycol (PEG). Initially, ovarian fragments (1 to 2 mm thick) and isolated secondary follicles were exposed to different concentrations (12.5, 25, 50 and 100%) of vitrification solution. Samples were exposed to the first two concentrations for 5 minutes at room temperature. Then, the fragments were exposed to the higher concentrations 15 minutes (fragments) or 5 minutes (isolated follicles) at 4 C. Following this step, samples (either ovarian fragments or isolated follicles) were placed on the surface of a metal cube partially submerged in liquid nitrogen and transferred to cryovials (10 follicles or 10 fragments each). Samples were kept in liquid nitrogen (-196 C) for 6 days Warming protocol
123 101 Cryovials containing samples of ovarian tissue and isolated secondary follicles were removed from liquid nitrogen and exposed to room temperature for 1 minute and immersed in a water bath (37 ºC) for 30 seconds. Soon after, it was performed the removal of cryoprotectants by immersion of ovarian fragments or secondary follicles in washing solutions composed by three baths of 5 minutes each with the solution of MEM plus 10% FBS and decreasing concentrations of sucrose (0.5, 0.25 and 0.0 M) in accordance with the protocol adapted from Lunardi et al. (2012) In vitro culture of sheep secondary follicles Secondary follicles were transferred into drops of 100 µl of culture medium under mineral oil in Petri-dishes (60 x 15 mm) and cultured for 18 days (one follicle/drop) at 39 C and 5% CO2 in air. Culture medium contained α-mem or TCM199, both supplemented with 10 µg/ml insulin, 5.5 µg/ml transferrin, 5 ng/ml selenium, 2 mm glutamine, 2 mm hypoxanthine, 3 mg/ml bovine serum albumin (BSA), 50 µg/ml ascorbic acid, 50 ng/ml leukemia inhibitory factor (LIF), 50 ng/ml Kit Ligant (KL) and 100 ng/ml follicle-stimulating hormone (FSH) Morphological analysis and assessment of in vitro follicular growth Before (day 0) and after 6, 12 and 18 days of in vitro culture, the percentage of morphologically normal follicles and follicular diameters from Control, Follicle-Vit and Tissue-Vit were determined. Follicles were classified as morphologically normal whether if they presented intact basement membrane, no extrusion of the cumulus-oocyte complexes (COCs) from the follicle, bright and homogeneous granulosa and theca cells. Follicle degeneration was recognized when the rupture of basement membrane, as well as oocytes and surrounding cells darkening, misshapen oocytes or decreased follicle diameter were observed. Follicular diameter was measured at the basement membrane (from the major and minor axes) of each follicle with the aid of an ocular micrometer inserted into a stereomicroscope (SMZ 645 Nikon, Tokyo, Japan; 100X). The average of these 2 measurements was used to determine the follicle diameter only in morphologically normal follicles. The daily mean increase in the follicular diameter (follicular growth rate) was calculated as the diameter of morphologically normal follicles at day 18 minus the diameter of the same follicle at day 0, divided by the total number of days in culture. Antral cavity formation was defined as a visible translucent cavity within the granulosa cell layers In vitro maturation (IVM) of COCs obtained from the cultured and in vivo grown Graafian follicles
124 102 At the end of the culture period, the COCs obtained from in vitro cultured were carefully harvested from intact follicles using 26G needles under a stereomicroscope. For a better comparison, an in vivo grown group was done. Therefore, in vivo-grown COCs were collected from antral ovarian follicles and only oocytes surrounded by at least one compact layer of cumulus cells were selected for IVM. The recovery rate of in vitro grown COCs was calculated by dividing the number of COCs by the number of viable follicles at day 18 of culture multiplied by 100. The selected COCs were washed in medium composed by TCM199 + HEPES (TCM199H) supplemented with pyruvate (0.911 mm/l) and 10% FBS followed by in vitro maturation medium. The maturation medium was composed by TCM199 + sodium bicarbonate (TCM199B) supplemented with 0.5 µg/ml recombinant bovine follicle-stimulating hormone (rbfsh) (NANOCORE, Campinas, São Paulo, Brazil), 5 µg/ml (luteinizing hormone) LH, 1 µg/ml 17βestradiol, 10 ng/ml recombinant epidermal growth factor (EGF), mm/l pyruvate, 100 µm/l cysteamine, 50 ng/ml recombinant insulin-like growth factor I (IGF-I) and 1% BSA. After washing, COCs were transferred to 50 µl drops of maturation medium under mineral oil and then incubated for 36 to 40 hours at 39 oc with 5% CO2 according to Luz et al. (2012) Assessment of oocyte viability and chromatin configuration After IVM, the COCs were denuded from surrounding expanded cumulus cells by manual pipetting in TCM199 HEPES containing 0.1% hyaluronidase and subjected to viability analysis. To this end, the oocytes were incubated in 100 µl drops of 2 µm ethidium homodimer-1 supplemented with 4 µm calcein-am, 0.5% glutaraldehyde and 10 µm Hoechst at room temperature for 30 min. After this, the oocytes were washed in PBS and were visualized under fluorescence microscopy (Nikon Eclipse 80i, Tokyo, Japan; 40 x). Oocytes were considered viable if the cytoplasm was positively stained with calcein-am (green) and not stained with ethidium homodimer-1 (red). The emitted fluorescent signals of calcein-am and ethidium homodimer - 1 were collected at 488 nm and 568 nm, respectively. In addition, oocytes were stained with Hoechst (Molecular Probes, Invitrogen, Karlsruhe, Germany) and then analyzed for chromatin configuration being emitted fluorescent signals at 483 nm. This dye was employed to analyze the oocyte s chromatin configuration through observation of the intact germinal vesicle (GV), meiotic resumption (including germinal vesicle breakdown - GVBD, metaphase I - MI, anaphase I - AI or telophase I - TI) or nuclear maturation (metaphase II - MII) Relative expression of Connexins 37, 43, BAX and BCL2 after in vitro culture preantral follicles
125 103 For this procedure, mural cells (granulosa and theca cells) from a total of 180 isolated secondary follicles (3 pools of 10 normal follicles from each treatment) were randomized into the following six treatments: 1) Control α-mem, 2) Control TCM199, 3) Follicle-Vit α-mem, 4) Follicle-Vit TCM199, 5) Tissue-Vit α-mem and 6) Tissue-Vit TCM199. For RNA isolation mural cells (Brito et al., 2015) were collected from each experimental treatment at 18 days of culture and stored in microcentrifuge tubes (1.5 ml) with Trizol, at -80 C until RNA extraction. Total RNA was isolated with the Trizol Plus Purification kit (Invitrogen, São Paulo, Brazil). The RNA preparations were treated with DNAse I and subjected to the RNeasy Micro kit (Invitrogen Life Technologies). All RNA samples were subjected to DNase I treatment with a PureLink DNase (Invitrogen, USA). RNA quality and concentration were determined using a Nano- Drop 2000 spectrophotometer (Thermo Scientific, USA). One unit of absorbance at 260 nm corresponded to 40 mg/ml RNA Reverse transcription PCR For reverse transcription, complementary DNA (cdna) was synthesized using 1 mg of RNA with SuperScript III Reverse Transcriptase (Invitrogen, Life Technologies, USA). Polymerase chain reactions were conducted in two steps. First, 1 mg of RNA, 50 ng/ml of random hexamer primers, 10-mM dntp mix, and diethyl pyrocarbonate-treatedwater (for a total volume of 13 ml) were heated to 65 C for 5 minutes and then immediately placed on ice for at least 1 minute. Then, 200 UI of SuperScript III RT, 10X RT buffer, 0.1-M DL-Dithiothreitol, and 40-UI RNaseOUT were added to the reaction mixture. Reverse transcription was performed under the following conditions: 25 C for 5 minutes, followed by 50 C for 40 minutes and finally, 70 C for 15 minutes. The first strand cdna was stored at 20 C. Real-time polymerase chain reaction (qpcr) was carried out using an icycler iq5 (Bio-Rad, USA). The reaction volume of 20 ml consisted of 5 ng of cdna, 1x Power SYBR Green PCR Master Mix, 10 µm of both forward and reverse primers, and ultrapure water. The qpcr protocol included an initial denaturation step at 95 C for 10 minutes, followed by 50 PCR cycles (15 seconds at 95 C, 1 minute at 60 C, 1 minute at 72 C) and a final extension step for 10 minutes at 72 C. The specificity of each primer set was determined using melt curve analysis, carried out between 60 C and 95 C for each primer pair. Fluorescence was initially monitored at 60 C, followed by subsequent measurements at 10-second intervals until the temperature reached 95 C. PPIA was used as a reference gene to normalize expression levels of the assayed genes. All samples were run in triplicate, and qpcrs were repeated at least twice. As negative controls, samples with reverse transcriptase but without RNA were used. The deltadeltacycle threshold (CT) method (Livak 2001) was used to transform CT values into normalized relative expression levels.
126 Primer design Gene sequences were obtained from the National Center for Biotechnology Information. Primers were designed according to the published Ovis aries Connexina 37, Connexina 43, BAX and BCL2 mrna sequences in GenBank, using the online free-access program, Primer3 (Table 1). The primers were tested for their specificity and efficiency using serial dilutions combining three different primer concentrations (10 mm, 5 mm, and 0.5 mm) with three cdna concentrations (5 ng, 0.5 ng, and 1 ng). The combination with the best results for specificity and efficiency (10 mmwith 5 ng) was used for the qpcrs Statistical analysis Data for discrete variables (morphologically normal follicles, antrum formation, recovery rate and chromatin configuration of oocytes) were analyzed as dispersion of frequency using Chisquare test. Otherwise, when the observed frequency was equal or less than five units, Exact Fisher s Test was applied. In both cases, results were expressed as percentages. Data for continuous variables (follicular diameter and growth rate after culture) Kruskall Wallis and Unpaired Test-t were initially evaluated for homocedasticity and normal distribution of the residues, by Bartlett s and Shapiro-Wilk tests, respectively. Confirmed both requirements underlying analysis of variance, the effects of treatment, time of culture and treatment by time interaction were analyzed using PROC MIXED of SAS (2002), including repeated statement to account for autocorrelation between sequential measurements. The model was Yijk=µ+Ri+Fj+Tk+(RT)ik+eijk, where Yijk is the observation of the jth follicle in the ith treatment at the kth time of culture, µ is the overall mean, Ri is the ith treatment, Fj is the random effect of the jth follicle within the ith treatment, Tk is the kth time of culture, (RT)ik is the treatment by time interaction term and eijk is the random residual effect. Comparisons among treatments or times were further analyzed by the Student-Newman-Keuls (SNK) test. A probability of P<0.05 indicated a significant difference and results were expressed as mean ± standard deviation (DP) (oocyte diameter) or expressed as mean ± standard error of the mean (SEM) (follicle diameter, growth rate). 3. Results 3.1. Morphologically normal follicles, extrusion and antral cavity formation rates during culture
127 105 A total of 412 secondary follicles were distributed randomly to treatments Control α-mem (n = 54), Control TCM199 (n = 62), Follicle-Vit α-mem (n = 68), Follicle-Vit TCM199 (n = 66), Tissue-Vit α-mem (n = 83), and Tissue-Vit TCM199 (n = 79) to in vitro culture for 18 days. The Control α-mem and TCM199 treatments showed similar percentage of morphologically normal follicles, however, lower when compared than vitrified follicles at the end of in vitro culture (Table 2). The α-mem Control (12.96%, n = 7/54) had percentage of degenerated follicles significantly higher to all treatments, except to Follicle-Vit TCM (3.03%, 2/66). The other treatments, Control TCM199 (1.59%, 1/63), Tissue-Vit α-mem (1.20%, 1/83) and Tissue-Vit TCM199 (1.27%) presented percentage of degenerated follicles similar to Follicle-Vit TCM199 and similar among them. On the other hand, the Follicle-Vit α-mem (0.00%, 0/68) presented no degenerated follicles after 18 days of in vitro culture. Control α-mem and Follicle-Vit (α-mem and TCM199), have percentage of antrum formation similar among them and higher when compared to Control TCM at the end of in vitro culture (P<0.05). The both treatments Tissue-Vit (α-mem and TCM199) have percentage of antrum formation similar between them and similar to Control (MEM and TCM199), although lower than both Follicle-Vit (α-mem and TCM199) at the end of in vitro culture (P<0.05) (Table 3) Follicular diameter and daily growth rate after in vitro culture At the end of culture Control (α-mem and TCM199) and Follicle-Vit TCM199 treatments had higher follicular diameters, with no difference among them. Significant higher follicular diameter at day 18 compared to day 0 of in vitro culture was observed in all treatments, except to Tissue-Vit α-mem (P<0.05) (Table 4). The Figure 2 illustrates the follicular daily growth rate in the treatments throughout the period of in vitro culture. The control groups were similar between them (α-mem: 7.86 ± 5.91 µm and TCM199: 8.18 ± 6.27 µm), but higher than vitrified groups. Among vitrified groups, the FollicleVit TCM199 (4.36 ± 2.29) was higher than Tissue-Vit α-mem (2.07 ± 1.54). On Figure 3, we can observe the higher percentage of follicles with decreased diameter in Control TCM199 (22.58%), Control α-mem (31.91%), Tissue-Vit α-mem (29.27%) and Tissue TCM199 (28.21%). However, lower percentage of follicles with decreased diameter in Follicle-Vit α-mem (16.18%) and FollicleVit TCM199 (10.94%) Relative expression of Connexins 37, 43, BAX and BCL2 of granulosa cells after in vitro cultured of secondary follicles
128 106 Although without significant difference, the relative expression of mrna for CX37 was observed only on Control in both media (α-mem and TCM199) (Figure 4). The data showed that mrna levels for connexins 43 did not vary (P > 0.05) between Controls (α-mem and TCM199). Similar results were observed among three vitrified treatments; however they were significantly lower than Control for both media. For CX43, the mrna levels were not detected in sufficient quantities on Follicle-Vit α-mem treatment in the present study (Figure 5). The relative gene expression of mrna was observed for BAX only on Control treatments (α-mem and TCM199), however without significant difference (Figure 6). When considering BCL2 gene, the relative expression was observed in all treatments, having higher expression in Tissue-Vit treatment in both media (α-mem and TCM199) when compared to other treatments, except to Follicle-Vit α-mem (Figure 7) Recovery rate, diameter, viability, and meiotic resumption of fresh or vitrified oocytes in vitro and in vivo-grown The recovery rate of oocytes from normal and intact cultured follicles was similar among all treatments (Control α-mem, Control TCM199, Follicle-Vit α-mem, Follicle-Vit TCM199, TissueVit α-mem, Tissue-Vit TCM199) (Table 5). The in vitro culture on both mediums (α-mem or TCM199) and the vitrification procedure of secondary follicles (isolated or enclosed on ovarian cortex), did not affected oocyte size after in vitro maturation (Table 6). After in vitro maturation, all oocytes were incubated with fluorescent labels calcein-am, ethidium homodimer-1 and Hoechst to evaluate viability and chromatin configuration, respectively. The treatments in vivo Grown, Control (α-mem and TCM199) and Follicle-Vit TCM199 showed the best percentage of oocyte viability (P<0.05). And the treatments in vivo Grown, Control (α-mem and TCM199), Follicle-Vit α-mem and Tissue-Vit α-mem showed the best percentage of meiotic resumption (P<0.05). The in vitro cultured oocytes from Control α-mem were surprising similar to the oocytes in vivo grown regarding to metaphase II rate. It is also noteworthy that the oocytes from Follicle-Vit α-mem and Control TCM199 presented similar percentage of metaphase II oocytes (Table 5). 4. Discussion: In this study, the follicular development, as well as the oocyte in vitro maturation were evaluated after two different vitrification procedures of sheep secondary follicles: isolated form (without surrounded stroma) or enclosed in stroma (within ovarian cortex). Furthermore, it was also
129 107 evaluated two different base medium (α-mem and TCM199) in a long term in vitro culture of fresh or vitrified secondary follicles and the meiosis resumption of their oocytes. The results of this study are scientific relevant to oncofertility area, due the vitrification of secondary follicles may provide an additional option for fertility preservation to female cancer patients, especially those who may have types of cancer that are not suitable for ovary transplantation. Vitrified follicles maintain intact/normal morphology better than follicles without previous vitrification (Control α-mem and TCM199). It is noteworthy that the follicles vitrified in an isolated form and cultured in α-mem medium (Follicle-Vit α-mem) presented no extruded follicles until day 18 of in vitro culture. This could be due the hardness of follicular membrane after vitrification procedure, limiting the oocytes release. The cryopreservation process may damage the structures of lipid bilayer which alters the movement of molecules and directly influence on its properties (Ghetler et al., 2005). In addition, the loss of the normal follicular morphology in other groups (Control α-mem, Control TCM199, Follicle-Vit TCM199, Tissue-Vit α-mem and TissueVit TCM199) occurred predominantly due the extrusion of oocytes and not by follicular degeneration. The Follicle-Vit treatments (α-mem and TCM199) presented antrum formation rate similar to Control α-mem and was higher than Tissue-Vit treatments (α-mem and TCM199). This can be due the preservation of granulosa cells was better on isolated and vitrified follicles compared to those vitrified enclosed on ovarian tissue. Thereafter, these cells had a better response to the FSH and growth factors (LIF and Kit Ligand) that were present on the culture media. Moreover, healthy granulosa cells showed greater permeability (Rodgers; Rodgers, 2010), which contributes to follicular fluid production and antrum formation. The follicles from Control groups (α-mem and TCM199) showed different rates of antrum formation. It is known that α-mem and TCM199 media are high variability in inorganic salts, amino acids, vitamins and other substances. However, the αmem contains Asparagin, L-Alanyl-L-Glutamine and in general has higher concentrations of amino acids and vitamins. In addition, the α-mem has pyruvate, the preferred source of nutrition of the oocyte.these characteristics among the two media could explain the observed variation on antrum cavity formation. The daily growth rate in all vitrified follicles was lower than Control α-mem and TCM199. The follicular growth occurs due the increase on the number and size of granulosa cells, as well as on the oocyte size. The hardening of follicular membrane combined with a possible reduction on granulosa cells proliferation due to vitrification process led to the observed reduction on follicular growth, once the oocyte diameter was similar among treatments. This study evaluated the gap junctions that are responsible for substances exchanges (inorganic salts, sugar, amino acids, nucleotides, vitamins, hormones and, secondary messengers as cyclic AMP and inositol triphosphate) essential to follicular and oocyte development. These
130 108 junctions are composed by proteins denominated conexins, which means inter-membrane channels that intermediate the cells-oocyte communication (Simon et al., 1997; Teilmann, 2005; Oyamada et al., 2013). A reduction on mrna expression of conexins 37 and 43 was observed on vitrified groups. Previous studies in mice (Boland; Gosden, 1994; Xu et al., 2009) and queens (Luciano et al, 2009; Tanpradit et al., 2015) also demonstrated similar results on the conexins 37 and 43 expression after the cryopreservation of preantral follicles. Similarly, our team in a recent study also showed that secondary follicles (oocyte and granulosa cells) vitrified on ovarian tissue with the ovarian tissue cryosystem (OTC) followed by isolation and in vitro culture during six days, only the CX43 expression was reduced while the CX37 expression remained unaffected (Silva et al.,non published data). The present study differed from our cited above due to different aspects as vitrification technique, media composition and in vitro culture period of secondary follicles. Moreover, the structure used to gene expression analysis was different since only mural cells were evaluated on this study. Regarding to the expression analysis of apoptosis genes (BAX e BCL2), only fresh follicles (Control α-mem and Control TCM199) showed detectable RNA expression for BAX. In contrast, other studies reported that vitrification process increased the expression of this gene (Taghavi et al., 2015; Jafarabadi et al., 2015; Du et al., 2015). The fresh follicles or those cultured in vitro can exhibit a high variation on BAX mrna expression (Chakravarthi et al. 2015). Such as the transcription and translation are extremely dynamic (Sales et al., 2014), we suggest that vitrification may have stimulated the transcription process so that all produced mrna was translated which made it impossible to obtain detectable levels for quantification. The treatments Follicle-Vit αmem e Tissue-Vit (α-mem and TCM199) showed a higher expression for BCL2 compared to control groups. These results are in accordance with a previous report which demonstrated an increased expression for BCL2 after vitrification (Jafarabadi et al. 2015). This can be a cell response in an attempt to overcome the apoptosis process usually observed as a consequence of the cryopreservation damage like the production of reactive oxygen (Grivicich, et al., 2007). Surprisingly, besides the fresh follicles cultured in α-mem have showed a similar rate of MII oocytes compared to the in vivo grown follicles, this parameter in isolated vitrified follicles cultured in α-mem (Follicle-Vit α-mem) was similar to that observed on fresh follicles cultured in TCM199. Usually, the in vitro maturation of in vivo developed oocytes is better than the in vitro developed oocytes (Hao et al., 2015). It can be explained due to the artificial conditions that in vitro developed follicles were exposed to maintain its growth and survival. In contrast, oocytes from in vivo developed follicles had the ideal conditions on its natural physiologic environment. Thus, it is not possible to predict which substances and their ideal combinations are fundamental to in vitro follicular development that would guarantee the best oocyte maturation rates.
131 109 In conclusion, we observed that the number of recovered oocytes after in vitro culture, as well as the oocyte diameter after in vitro maturation was not affected by the vitrification neither by the base media used for follicular culture. Although the vitrification process reduced the gene expression for CX37, CX43 e BAX, the high expression for BCL2 on vitrified follicles may be a sign of overcoming the apoptosis process on these follicles. Unlike the follicles that were vitrified enclosed on ovarian tissue, the most part of secondary follicles vitrified isolated from tissue presented antrum formation. In addition, after vitrification only oocytes from isolated follicles and cultured in α-mem achieved the MII stage. 5. Acknowledgements: This work was supported by CNPq. Franciele Osmarini Lunardi is a recipient of a grant from CAPES Brazil. In addition, Ana Paula Ribeiro Rodrigues and José Ricardo de Figueiredo are recipients of a grant from CNPq Brazil. Johan Smitz is Especial Visitor Researcher from CAPES.
132 110 Table 1. Oligonucleotide primers used for the real-time polymerase chain reaction analysis of ovine follicles. Target gene PPIA CX37a CX43a BAX BCL2 Primer sequence (5 3 ) TCATTTGCACTGCCAAGACTG TCATCGCCTCTTTCACTTTGC CGACGAGCAGTCGGATTT AGATGACATGGCCCAGGTAG ATGAGCAGTCTGCCTTTCGT TCTGCTTCAAGTGCATGTCC AACATGGAGCTGCAGAGGAT TCGAAGGAAGTCCAATGTCC ATG TGT GTC GAG AGC GTC AA GCC AGG AGA AAT CAA ACA GG Sense Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse GenBank reference XM_ AY AY XM_ XM_ Table 2. Percentage of isolated morphologically normal sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit α-mem and Tissue-Vit TCM199) at days 0, 6, 12, and 18 of in vitro culture. Control MEM Control TCM Aa % (68/68) Aa Follicle-Vit TCM % (66/66) Aa Tissue-Vit MEM % (83/83) Aa Tissue-Vit TCM % (79/79)Aa Day % (54/54) Day % (53/54)Aa % (62/62)Aa % (68/68)Aa 98.48% (65/66)Aa 98.80% (82/83)Aa % (79/79)Aa Day % (44/54)Bb 90.32% (56/62)Ba % (68/68)Aa 98.48% (65/66)Aa 98.80% (82/83)Aa 98.73% (78/79)Aa Day % (30/54)Bc 72.58% (45/62)Bb % (68/68)Aa 96.97% (64/66)Aa 98.80% (82/83)Aa 98.73% (78/79)Aa AB % (62/62) Follicle-Vit MEM Aa Different uppercase letters indicate statistically significant differences among groups (columns) (P<0.05) within the same day. lowercase letters indicate statistically significant differences among days of culture (rows) (P<0.05) within the same group. a,b Different
133 111 Table 3. Percentage of antrum formation of sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit α-mem and Tissue-Vit TCM199) at days 6, 12, and 18 of in vitro culture. Control MEM Control TCM Follicle-Vit MEM Follicle-Vit TCM Tissue-Vit MEM Tissue-Vit TCM Day % (0/47) 0.00% (0/62) 0.00% (0/68) 0.00% (0/64) 0.00% (0/82) 0.00% (0/78) Day % (14/47) BCb 22.58% (14/62) Cb 44.12% (30/68) ABc 53.13% (34/64) Ab 43.90% (36/82) ABc 42.31% (33/78) ABb Day % (36/47) Aa 66.13% (41/62) ABa 73.53% (50/68) Ab 68.75% (44/64) ABb 64.63% (53/82) ABb 56.41% (44/78) Bab Day % (40/47) ABa 67.74% (42/62) Ca 92.65% (63/68) Aa 92.19% (59/64) Aa 80.49% (66/82) BCa 70.51% (55/78) BCa A,B,C Different uppercase letters indicate statistically significant differences among groups (columns) (P<0.05) within the same day. a,b,c Different lowercase letters indicate statistically significant differences among days of culture (rows) (P<0.05) within the same group. Table 4. Follicular diameter (µm) (mean ± SEM) of sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit α-mem and Tissue-Vit TCM199) at days 0, 6, 12, and 18 of in vitro culture. Control MEM Control TCM Follicle-Vit MEM Follicle-Vit TCM Tissue-Vit MEM Tissue-Vit TCM Day ± Ac ± Ab ± Ab ± Ab ± Aa ± Ab Day ± Abc ± Ab ± Aa ± Aa ± Aa ± Aab Day ± Aab ± Aa ± Ba ± ABa ± Ba ± ABab Day ± Aa ± Aa ± Ba ± ABa ± Ba ± Ba AB Different uppercase letters indicate statistically significant differences among groups (columns) (P<0.05) within the same day. lowercase letters indicate statistically significant differences among days of culture (rows) (P<0.05) within the same group. a,b,c Different
134 112 Table 5. Oocyte recovery rate (%), viability (%) and meiotic stages (%) of sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit α-mem and TissueVit TCM199). No of oocytes Groups recovered / No cultured follicles (%) No of calcein AM Cromatin No of oocytes with GVBD/ No of MI/ No of oocytes MII/ No of oocytes viable oocytes / No degradation/ No of meiosis resumption / oocytes with with meiosis with meiosis o of oocytes (%) oocytes (%) N of viable oocytes meiosis resumption resumption resumption (%) In Vivo Grown - 89,29% (50/56) ab 12.00% (6/56) a 80.00% (40/50) a 32.50% (13/40) b 12.50% (5/40) a 55.00% (22/40) a Control MEM 31.48% (17/54) a % (17/17) a 0.00% (0/17) 76.47% (13/17) a 46.15% (6/13) ab 23.08% (3/13) a 30.77% (4/13) ab Control TCM 22.58% (14/62) a 92.86% (13/14) ab 7.14% (1/14) a 53.85% (7/13) ab 57.14% (4/7) ab 28.57% (2/7) a 14.29% (1/7) b Follicle-Vit MEM 29.41% (20/68) a 75.00% (15/20) bc 20.00% (4/20) a 66.67% (10/15) a 70.00% (7/10) a 10.00% (1/10) a 20.00% (2/10) b Follicle-Vit TCM 31.82% (21/66) a 85.71% (18/21) ab 14.29% (3/21) a 22.22% (4/18) bc % (4/4) a 0.00% (0/4) 0.00% (0/4) Tissue-Vit MEM 26.51% (22/83) a 45.45% (10/22) c 13.64% (3/22) a 60.00% (6/10) a 83.33% (5/6) a 16.67% (1/6) a 0.00% (0/6) Tissue-Vit TCM 34.18% (27/79) a 70.37% (19/27) bc 11.11% (3/27) a 15.79% (3/19) c % (3/3) a 0.00% (0/3) 0.00% (0/3) a,b,c,d Different lowercase letters indicate statistically significant differences (P<0.05) among groups (rows).
135 113 Table 6. Oocyte diameter (µm ± SD) of sheep preantral follicles without vitrification (Control α-mem and Control TCM199) and after vitrification of isolated follicles (Follicle-Vit α-mem and Follicle-Vit TCM199) or ovarian tissue (Tissue-Vit α-mem and Tissue-Vit TCM199) after 18 days of in vitro culture and in vitro maturation. a Treatments Oocyte diamter (µm ± SD) In Vivo Grown ± a Control MEM ± a Control TCM ± a Follicle-Vit MEM ± 9.43 a Follicle-Vit TCM ± a Tissue-Vit MEM ± a Tissue-Vit TCM ± a Different lowercase letters indicate statistically significant differences (P<0.05) among groups (rows).
136 114 Figures Figure 1. Experimental design for vitrification and in vitro culture of sheep secondary follicles. SF: secondary follicles. IVC: in vitro culture. IVM: in vitro maturation.
137 115 Figure 2. Daily growth rate (µm/day) (mean ± SEM) of sheep preantral follicles without vitrification (Control α-mem, n = 32/47; and Control TCM199, n = 48/62) and after vitrification of isolated follicles (Follicle-Vit MEM, n = 57/68; and Follicle-Vit TCM199, n = 57/64) or ovarian tissue (TissueVit α-mem, n = 58/82; and Tissue-Vit TCM199, n = 56/78) after 18 days of in vitro culture. A,B,C Different uppercase letters indicate statistically significant differences among groups (P<0.05). Figure 3. Percentage of sheep preantral follicles with decreased diameter in groups without vitrification (Control α-mem, n = 15/47; and Control TCM199, n = 14/62) and after vitrification of isolated follicles (Follicle-Vit α-mem, n = 11/68; and Follicle-Vit TCM, n = 7/64) or ovarian tissue (Tissue-Vit α-mem, n = 24/82; and Tissue-Vit TCM199, n = 22/78) after 18 days of culture. A,B,C Different uppercase letters indicate statistically significant differences among groups (P<0.05).
EFEITO DO TIPO DE SISTEMAS DE CULTIVO NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS ISOLADOS
 1 EFEITO DO TIPO DE SISTEMAS DE CULTIVO NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS ISOLADOS EFFECT OF TYPE OF CULTURE SYSTEM ON THE IN VITRO DEVELOPMENT OF ISOLATED CAPRINE PREANTRAL
1 EFEITO DO TIPO DE SISTEMAS DE CULTIVO NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS ISOLADOS EFFECT OF TYPE OF CULTURE SYSTEM ON THE IN VITRO DEVELOPMENT OF ISOLATED CAPRINE PREANTRAL
GAMETOGÊNESE DIFERENCIAÇÃO DOS GAMETAS. Profª Msc. Tatiane da Silva Poló
 GAMETOGÊNESE DIFERENCIAÇÃO DOS GAMETAS Profª Msc. Tatiane da Silva Poló ou OOGÊNESE ou OVULOGÊNESE e FOLICULOGÊNESE DEFINIÇÃO OVOGÊNESE: É uma sequência de eventos através dos quais as células germinativas
GAMETOGÊNESE DIFERENCIAÇÃO DOS GAMETAS Profª Msc. Tatiane da Silva Poló ou OOGÊNESE ou OVULOGÊNESE e FOLICULOGÊNESE DEFINIÇÃO OVOGÊNESE: É uma sequência de eventos através dos quais as células germinativas
Eixo hipotalamico-hipofisario-gonadal na femea Funcoes dos hormonios Organizacao das gonadas e desenvolvimento folicular Ciclo estral da vaca
 Topicos Eixo hipotalamico-hipofisario-gonadal na femea Funcoes dos hormonios Organizacao das gonadas e desenvolvimento folicular Ciclo estral da vaca Fertilizacao e desenvolvimento embrionario Cerebro
Topicos Eixo hipotalamico-hipofisario-gonadal na femea Funcoes dos hormonios Organizacao das gonadas e desenvolvimento folicular Ciclo estral da vaca Fertilizacao e desenvolvimento embrionario Cerebro
Competência Oocitária
 Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Laboratório de Reprodução Animal -EMBRAPA Competência Oocitária Lucas Hax Médico Veterinário Doutorando em Biotecnologia
Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Laboratório de Reprodução Animal -EMBRAPA Competência Oocitária Lucas Hax Médico Veterinário Doutorando em Biotecnologia
SISTEMA REPRODUTOR FEMININO
 Universidade Estadual do Ceará Faculdade de Veterinária SISTEMA REPRODUTOR FEMININO Histologia e Embriologia Geral Veterinária Profa. Janaina Serra Azul Monteiro Evangelista SISTEMA REPRODUTOR FEMININO
Universidade Estadual do Ceará Faculdade de Veterinária SISTEMA REPRODUTOR FEMININO Histologia e Embriologia Geral Veterinária Profa. Janaina Serra Azul Monteiro Evangelista SISTEMA REPRODUTOR FEMININO
Bloco X- Testes- Células germinativas
 Bloco X- Testes- Células germinativas 1) Das células progenitoras dos gametas podemos afirmar: I. São haplóides e migram para as gônadas em desenvolvimento II. São chamadas células germinativas primordiais
Bloco X- Testes- Células germinativas 1) Das células progenitoras dos gametas podemos afirmar: I. São haplóides e migram para as gônadas em desenvolvimento II. São chamadas células germinativas primordiais
Expressão Gênica em Oócitos e Embriões
 Universidade Federal de Pelotas Graduação em Biotecnologias Manipulação de Gametas e Embriões Expressão Gênica em Oócitos e Embriões Prof. Tiago Collares Prof a. Priscila M. M. de Leon Janeiro, 2013 Análise
Universidade Federal de Pelotas Graduação em Biotecnologias Manipulação de Gametas e Embriões Expressão Gênica em Oócitos e Embriões Prof. Tiago Collares Prof a. Priscila M. M. de Leon Janeiro, 2013 Análise
Produção in vitro de embriões
 Universidade Estadual do Ceará Faculdade de Veterinária Programa de Pós-Graduação em Ciências Veterinárias Biotécnicas Aplicadas à Reprodução de Fêmeas Caprinas e Ovinas Produção in vitro de embriões Vicente
Universidade Estadual do Ceará Faculdade de Veterinária Programa de Pós-Graduação em Ciências Veterinárias Biotécnicas Aplicadas à Reprodução de Fêmeas Caprinas e Ovinas Produção in vitro de embriões Vicente
Produção in vitro de embriões ovinos
 NUPEEC Núcleo de Pesquisa, Ensino e Extensão em Pecuária Produção in vitro de embriões ovinos Apresentadores: Mauri Mazurek Rodrigo C. de C. de Azambuja Orientação: Liziane Lemos Viana Small Ruminant Resarch
NUPEEC Núcleo de Pesquisa, Ensino e Extensão em Pecuária Produção in vitro de embriões ovinos Apresentadores: Mauri Mazurek Rodrigo C. de C. de Azambuja Orientação: Liziane Lemos Viana Small Ruminant Resarch
Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária
 Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Efeito do ácido lisofosfatídico durante a maturação in vitro do complexo cumulus-oócito: expansão do cumulus, metabolismo
Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Efeito do ácido lisofosfatídico durante a maturação in vitro do complexo cumulus-oócito: expansão do cumulus, metabolismo
O sistema reprodutor feminino. Os ovários e os órgãos acessórios. Aula N50
 O sistema reprodutor feminino. Os ovários e os órgãos acessórios. Aula N50 Sistema reprodutor feminino Ovários = produz óvulos Tubas uterinas = transportam e protegem os óvulos Útero = prove meio adequado
O sistema reprodutor feminino. Os ovários e os órgãos acessórios. Aula N50 Sistema reprodutor feminino Ovários = produz óvulos Tubas uterinas = transportam e protegem os óvulos Útero = prove meio adequado
Gametogênese Prof. Dr. Philip Wolff Prof. Dr. Renato M. Salgado
 Prof. Dr. Philip Wolff Prof. Dr. Renato M. Salgado A gametogênese é o processo de produção dos gametas, as células reprodutoras. Esta produção ocorre nas gônadas e seu principal evento é a meiose, responsável
Prof. Dr. Philip Wolff Prof. Dr. Renato M. Salgado A gametogênese é o processo de produção dos gametas, as células reprodutoras. Esta produção ocorre nas gônadas e seu principal evento é a meiose, responsável
Criopreservação de Embriões
 Universidade Estadual do Ceará Faculdade de Veterinária Biotecnologia da Reprodução Animal Criopreservação de Embriões Vicente José de F. Freitas Laboratório de Fisiologia e Controle da Reprodução www.uece.br/lfcr
Universidade Estadual do Ceará Faculdade de Veterinária Biotecnologia da Reprodução Animal Criopreservação de Embriões Vicente José de F. Freitas Laboratório de Fisiologia e Controle da Reprodução www.uece.br/lfcr
UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS DOUTORADO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS DOUTORADO EM CIÊNCIAS VETERINÁRIAS HUDSON HENRIQUE VIEIRA CORREIA CULTIVO IN VITRO DE OÓCITOS
UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS DOUTORADO EM CIÊNCIAS VETERINÁRIAS HUDSON HENRIQUE VIEIRA CORREIA CULTIVO IN VITRO DE OÓCITOS
REGULAÇÃO DA PRODUÇÃO DO HORMÔNIO ANTI- MÜLLERIANO EM VACAS: UM ESTUDO COMPARATIVO EM NÍVEL ENDÓCRINO, OVARIANO, FOLÍCULAR E DE CÉLULAS DA GRANULOSA
 REGULAÇÃO DA PRODUÇÃO DO HORMÔNIO ANTI- MÜLLERIANO EM VACA: UM ETUDO COMPARATIVO EM NÍVEL ENDÓCRINO, OVARIANO, FOLÍCULAR E DE CÉLULA DA GRANULOA Douglas Peraoli & Francielle Bado Augusto chneider FI: 3.300
REGULAÇÃO DA PRODUÇÃO DO HORMÔNIO ANTI- MÜLLERIANO EM VACA: UM ETUDO COMPARATIVO EM NÍVEL ENDÓCRINO, OVARIANO, FOLÍCULAR E DE CÉLULA DA GRANULOA Douglas Peraoli & Francielle Bado Augusto chneider FI: 3.300
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 0 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS NATHALIE JIATSA DONFACK AUTO E XENOTRANSPLANTE PARA
0 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS NATHALIE JIATSA DONFACK AUTO E XENOTRANSPLANTE PARA
Efeito da desnutrição materna durante a gestação no desenvolvimento ovariano inicial e subsequente desenvolvimento folicular em fetos de ovelhas
 Universidade Federal de Pelotas Faculdade de Veterinária Núcleo de Pesquisa Ensino e Extensão em Pecuária Efeito da desnutrição materna durante a gestação no desenvolvimento ovariano inicial e subsequente
Universidade Federal de Pelotas Faculdade de Veterinária Núcleo de Pesquisa Ensino e Extensão em Pecuária Efeito da desnutrição materna durante a gestação no desenvolvimento ovariano inicial e subsequente
Criopreservação de Embriões
 UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA BIOTECNOLOGIA DA REPRODUÇÃO ANIMAL Criopreservação de Embriões Vicente José de F. Freitas Laboratório de Fisiologia e Controle da Reprodução www.uece.br/lfcr
UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA BIOTECNOLOGIA DA REPRODUÇÃO ANIMAL Criopreservação de Embriões Vicente José de F. Freitas Laboratório de Fisiologia e Controle da Reprodução www.uece.br/lfcr
Regulação da foliculogênese e determinação da taxa de ovulação em ruminantes
 Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Regulação da foliculogênese e determinação da taxa de ovulação em ruminantes Lucas Hax e Patrícia Mattei Pelotas, 18 de
Universidade Federal de Pelotas Núcleo de Pesquisa, Ensino e Extensão em Pecuária Regulação da foliculogênese e determinação da taxa de ovulação em ruminantes Lucas Hax e Patrícia Mattei Pelotas, 18 de
Efeito de dois diferentes protocolos para congelação lenta de embriões bovinos produzidos in vitro na região da Amazônia Legal
 Efeito de dois diferentes protocolos para congelação lenta de embriões bovinos produzidos in vitro na região da Amazônia Legal Karina Almeida Maciel 1 ; Márcio Gianordoli Teixeira Gomes 2 ; Francisca Elda
Efeito de dois diferentes protocolos para congelação lenta de embriões bovinos produzidos in vitro na região da Amazônia Legal Karina Almeida Maciel 1 ; Márcio Gianordoli Teixeira Gomes 2 ; Francisca Elda
DOAÇÃO DE ÓVULOS: BANCO DE OVÓCITOS LEVA REALMENTE AOS MESMO RESULTADOS DE DOAÇÃO A FRESCO? Daniela Paes de Almeida Ferreira Braga, DVM, M.Sc.
 DOAÇÃO DE ÓVULOS: BANCO DE OVÓCITOS LEVA REALMENTE AOS MESMO RESULTADOS DE DOAÇÃO A FRESCO? Daniela Paes de Almeida Ferreira Braga, DVM, M.Sc. DOAÇÃO DE ÓVULOS OVODOAÇÃO Desordens genéticas hereditárias
DOAÇÃO DE ÓVULOS: BANCO DE OVÓCITOS LEVA REALMENTE AOS MESMO RESULTADOS DE DOAÇÃO A FRESCO? Daniela Paes de Almeida Ferreira Braga, DVM, M.Sc. DOAÇÃO DE ÓVULOS OVODOAÇÃO Desordens genéticas hereditárias
Gametogênese ESPERMATOGÊNESE. Prof a. Marta G. Amaral, Dra. Embriologia molecular
 Gametogênese ESPERMATOGÊNESE Prof a. Marta G. Amaral, Dra. Embriologia molecular Gametogênese CGP se formam no epiblasto na 2ª sem. Na 4ª sem. migram do o saco vitelino para as gônadas em desenvolvimento.
Gametogênese ESPERMATOGÊNESE Prof a. Marta G. Amaral, Dra. Embriologia molecular Gametogênese CGP se formam no epiblasto na 2ª sem. Na 4ª sem. migram do o saco vitelino para as gônadas em desenvolvimento.
Ciclo de Vida Celular. Professora: Luciana Ramalho 2017
 Ciclo de Vida Celular Professora: Luciana Ramalho 2017 Tipos de células Procarionte Sem núcleo Ex: Bactérias Eucarionte Com núcleo Ex: Vegetal, Protozoário, Animal e Fungos Exercício (FEI) Uma célula procarionte
Ciclo de Vida Celular Professora: Luciana Ramalho 2017 Tipos de células Procarionte Sem núcleo Ex: Bactérias Eucarionte Com núcleo Ex: Vegetal, Protozoário, Animal e Fungos Exercício (FEI) Uma célula procarionte
Criopreservação de embriões
 Laboratório de Fisiologia e Controle da Reprodução Dr. Ribrio Ivan Tavares Pereira Batista 09/10/2014 SUMÁRIO 1. INTRODUÇÃO 2. PRINCÍPIOS DA CRIOPRESERVAÇÃO 3. TÉCNICAS DE CRIOPRESERVAÇÃO DE EMBRIÕES 4.
Laboratório de Fisiologia e Controle da Reprodução Dr. Ribrio Ivan Tavares Pereira Batista 09/10/2014 SUMÁRIO 1. INTRODUÇÃO 2. PRINCÍPIOS DA CRIOPRESERVAÇÃO 3. TÉCNICAS DE CRIOPRESERVAÇÃO DE EMBRIÕES 4.
Coleta, Classificação e Maturação in vitrode Oócitos
 Universidade Federal de Pelotas Graduação em Biotecnologias Disciplina de Manipulação de Gametas e Embriões 2ª Aula: Coleta, Classificação e Maturação in vitrode Oócitos Priscila M. M. de Leon Doutoranda
Universidade Federal de Pelotas Graduação em Biotecnologias Disciplina de Manipulação de Gametas e Embriões 2ª Aula: Coleta, Classificação e Maturação in vitrode Oócitos Priscila M. M. de Leon Doutoranda
UNIVERSIDADE ESTADUAL DO CEARÁ-UECE FACULDADE DE VETERINÁRIA-FAVET PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS- PPGCV
 1 UNIVERSIDADE ESTADUAL DO CEARÁ-UECE FACULDADE DE VETERINÁRIA-FAVET PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS- PPGCV PATRÍCIA MAGALHÃES DE ANDRADE EFEITO DOS MEIOS DE CULTIVO DE BASE α-mem E
1 UNIVERSIDADE ESTADUAL DO CEARÁ-UECE FACULDADE DE VETERINÁRIA-FAVET PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS- PPGCV PATRÍCIA MAGALHÃES DE ANDRADE EFEITO DOS MEIOS DE CULTIVO DE BASE α-mem E
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCISCA TUELLY BANDEIRA DE OLIVEIRA ANÁLISE DO DESENVOLVIMENTO
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCISCA TUELLY BANDEIRA DE OLIVEIRA ANÁLISE DO DESENVOLVIMENTO
NUPEEC. Núcleo de Pesquisa, Ensino e Extensão em Pecuária
 NUPEEC Núcleo de Pesquisa, Ensino e Extensão em Pecuária Fatores Parácrinos, Autócrinos e Endócrinos que Mediam a Influência da Nutrição na Reprodução em Bovinos e Suínos Journal of Animal Science 1996
NUPEEC Núcleo de Pesquisa, Ensino e Extensão em Pecuária Fatores Parácrinos, Autócrinos e Endócrinos que Mediam a Influência da Nutrição na Reprodução em Bovinos e Suínos Journal of Animal Science 1996
Fisiologia da Reprodução
 Texto de Referência Fisiologia da Reprodução Conhecimentos de anatomia e fisiologia são imprescindíveis para o Médico Veterinário que pretende trabalhar de forma correta e tirar o máximo proveito da técnica
Texto de Referência Fisiologia da Reprodução Conhecimentos de anatomia e fisiologia são imprescindíveis para o Médico Veterinário que pretende trabalhar de forma correta e tirar o máximo proveito da técnica
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS SIMONE VIEIRA CASTRO EFEITO DO MÉTODO DE CRIOPRESERVAÇÃO
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS SIMONE VIEIRA CASTRO EFEITO DO MÉTODO DE CRIOPRESERVAÇÃO
ENDOCRINOLOGIA REPRODUTIVA. M. V. Doutorando Lucas Balinhas Farias
 ENDOCRINOLOGIA REPRODUTIVA M. V. Doutorando Lucas Balinhas Farias Anatomia do sistema reprodutivo feminino Genitália externa, vagina, cérvix, útero, ovidutos e ovários Maturidade Sexual É representada
ENDOCRINOLOGIA REPRODUTIVA M. V. Doutorando Lucas Balinhas Farias Anatomia do sistema reprodutivo feminino Genitália externa, vagina, cérvix, útero, ovidutos e ovários Maturidade Sexual É representada
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS CLEIDSON MANOEL GOMES DA SILVA UTILIZAÇÃO DO HORMÔNIO
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS CLEIDSON MANOEL GOMES DA SILVA UTILIZAÇÃO DO HORMÔNIO
BIOLOGIA. Moléculas, Células e Tecidos Gametogênese. Prof. Daniele Duó
 BIOLOGIA Moléculas, Células e Tecidos https://www.qconcursos.com Prof. Daniele Duó - A reprodução sexuada começa com a formação dos gametas, denominado gametogênese. espermatogênese ovulogênese ou ovogênese
BIOLOGIA Moléculas, Células e Tecidos https://www.qconcursos.com Prof. Daniele Duó - A reprodução sexuada começa com a formação dos gametas, denominado gametogênese. espermatogênese ovulogênese ou ovogênese
UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS MESTRADO ACADÊMICO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS MESTRADO ACADÊMICO EM CIÊNCIAS VETERINÁRIAS LIVIA BRUNETTI APOLLONI EFEITO DO HORMÔNIO ANDROSTENEDIONA
UNIVERSIDADE ESTADUAL DO CEARÁ FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS MESTRADO ACADÊMICO EM CIÊNCIAS VETERINÁRIAS LIVIA BRUNETTI APOLLONI EFEITO DO HORMÔNIO ANDROSTENEDIONA
GAMETOGÊNESE ESPERMATOGÊNESE OVOLUGÊNESE
 BIOLOGIA FRENTE A SÉRIE: 9º ANO GAMETOGÊNESE ESPERMATOGÊNESE OVOLUGÊNESE Profª. LUCIANA ARAUJO MEIOSE E GAMETAS É por meio da divisão meiótica que as células da linhagem germinativa fabricam os gametas,
BIOLOGIA FRENTE A SÉRIE: 9º ANO GAMETOGÊNESE ESPERMATOGÊNESE OVOLUGÊNESE Profª. LUCIANA ARAUJO MEIOSE E GAMETAS É por meio da divisão meiótica que as células da linhagem germinativa fabricam os gametas,
Reprodução. Biologia Ambiental Professora: Keffn Arantes
 Reprodução Biologia Ambiental Professora: Keffn Arantes GAMETAS E FECUNDAÇÃO NOS ANIMAIS *MEIOSE-significa diminuição. *Seres humanos têm 46 cromossomos nas células corporais. *Gametas apresentam
Reprodução Biologia Ambiental Professora: Keffn Arantes GAMETAS E FECUNDAÇÃO NOS ANIMAIS *MEIOSE-significa diminuição. *Seres humanos têm 46 cromossomos nas células corporais. *Gametas apresentam
GAMETOGÊNESE. Processo de formação e desenvolvimento das células germinativas especializadas OS GAMETAS.
 GAMETOGÊNESE 1 GAMETOGÊNESE Processo de formação e desenvolvimento das células germinativas especializadas OS GAMETAS. Gameta masculino Espermatozóide. Gameta feminino Ovócito. Os gametas possuem metade
GAMETOGÊNESE 1 GAMETOGÊNESE Processo de formação e desenvolvimento das células germinativas especializadas OS GAMETAS. Gameta masculino Espermatozóide. Gameta feminino Ovócito. Os gametas possuem metade
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ÉRICA SUZANNE SOARES LEAL DESENVOLVIMENTO IN VITRO
1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ÉRICA SUZANNE SOARES LEAL DESENVOLVIMENTO IN VITRO
ALLANA FERREIRA DA COSTA PESSOA
 1 UNIVERSIDADE ESTADUAL DO CEARÁ- UECE PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA- FAVET PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS-PPGCV ALLANA FERREIRA DA COSTA PESSOA
1 UNIVERSIDADE ESTADUAL DO CEARÁ- UECE PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA- FAVET PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS-PPGCV ALLANA FERREIRA DA COSTA PESSOA
Aparelho Reprodutor Feminino
 Pontifícia Universidade Católica de Goiás Departamento de Biologia Aparelho Reprodutor Feminino Prof. Msc. Macks Wendhell Gonçalves mackswenedhell@gmail.com É formado por: Parte interna: - Dois ovários
Pontifícia Universidade Católica de Goiás Departamento de Biologia Aparelho Reprodutor Feminino Prof. Msc. Macks Wendhell Gonçalves mackswenedhell@gmail.com É formado por: Parte interna: - Dois ovários
Produção in vitro de embriões
 Universidade Estadual do Ceará Faculdade de Veterinária Programa de Pós- Graduação em Ciências Veterinárias Biotécnicas Aplicadas à Reprodução de Fêmeas Caprinas e Ovinas Produção in vitro de embriões
Universidade Estadual do Ceará Faculdade de Veterinária Programa de Pós- Graduação em Ciências Veterinárias Biotécnicas Aplicadas à Reprodução de Fêmeas Caprinas e Ovinas Produção in vitro de embriões
hormônios do sistema genital masculino gônadas masculinas
 Gônadas masculinas Os hormônios do sistema genital masculino são produzidos nas gônadas masculinas, muito conhecidas como testículos. São os hormônios que determinam as características sexuais secundárias,
Gônadas masculinas Os hormônios do sistema genital masculino são produzidos nas gônadas masculinas, muito conhecidas como testículos. São os hormônios que determinam as características sexuais secundárias,
Divisão celular. A única situação em que dividir e multiplicar significam a mesma coisa
 Divisão celular A única situação em que dividir e multiplicar significam a mesma coisa Todos as células se dividem: 1. Em organismos unicelulares, divisão celular é o modo principal de reprodução 2. Em
Divisão celular A única situação em que dividir e multiplicar significam a mesma coisa Todos as células se dividem: 1. Em organismos unicelulares, divisão celular é o modo principal de reprodução 2. Em
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 1 l UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ISADORA MACHADO TEIXEIRA LIMA DESENVOLVIMENTO IN
1 l UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ISADORA MACHADO TEIXEIRA LIMA DESENVOLVIMENTO IN
Gametogênese Ovogênese. Prof a. Marta G. Amaral, Dra. Embriologia molecular
 Gametogênese Ovogênese Prof a. Marta G. Amaral, Dra. Embriologia molecular Cél germinativa primordial (CGP) CGP Oogônias Inicia antes do nascimento Depois entra em repouso Na puberdade termina a diferenciação
Gametogênese Ovogênese Prof a. Marta G. Amaral, Dra. Embriologia molecular Cél germinativa primordial (CGP) CGP Oogônias Inicia antes do nascimento Depois entra em repouso Na puberdade termina a diferenciação
BIOLOGIA. Moléculas, células e tecidos. Gametogênese Parte 2. Professor: Alex Santos
 BIOLOGIA Moléculas, células e tecidos Parte 2 Professor: Alex Santos Tópicos em abordagem: Parte 2 Ovocitogênese: I A Ovocitogênese; II Quadro comparativo de gametogêneses; I Ovocitogênese: 1.1 Visão geral:
BIOLOGIA Moléculas, células e tecidos Parte 2 Professor: Alex Santos Tópicos em abordagem: Parte 2 Ovocitogênese: I A Ovocitogênese; II Quadro comparativo de gametogêneses; I Ovocitogênese: 1.1 Visão geral:
Biologia Celular. A Célula. Células 02/23/2008. Membrana Celular. Citoplasma. Eucariotes. Procariotes
 Biologia Celular Células Procariotes Procariotes são organismos unicelulares, presentes em todos os meios ambientes. Constituem o maior grupo de organismos da natureza, principalmente porque as bactérias
Biologia Celular Células Procariotes Procariotes são organismos unicelulares, presentes em todos os meios ambientes. Constituem o maior grupo de organismos da natureza, principalmente porque as bactérias
Ciclo Celular. Dividido em: - Intérfase - Divisão celular: Dois tipos fundamentais. Mitose Meiose
 Meiose Ciclo Celular Dividido em: - Intérfase - Divisão celular: Dois tipos fundamentais Mitose Meiose Antes de qualquer divisão celular há duplicação do DNA durante a intérfase. Divisão Celular O Ciclo
Meiose Ciclo Celular Dividido em: - Intérfase - Divisão celular: Dois tipos fundamentais Mitose Meiose Antes de qualquer divisão celular há duplicação do DNA durante a intérfase. Divisão Celular O Ciclo
WALTER ARTHUR SILVA VALENTE
 WALTER ARTHUR SILVA VALENTE ANÁLISE IMUNOISTOQUÍMICA DA EXPRESSÃO DE Ki-67, Bcl-2, EGF e CD57 NO ESPECTRO EVOLUTIVO DE GRANULOMAS E CISTOS PERIRRADICULARES 2016 i WALTER ARTHUR SILVA VALENTE ANÁLISE IMUNOISTOQUÍMICA
WALTER ARTHUR SILVA VALENTE ANÁLISE IMUNOISTOQUÍMICA DA EXPRESSÃO DE Ki-67, Bcl-2, EGF e CD57 NO ESPECTRO EVOLUTIVO DE GRANULOMAS E CISTOS PERIRRADICULARES 2016 i WALTER ARTHUR SILVA VALENTE ANÁLISE IMUNOISTOQUÍMICA
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCIELE OSMARINI LUNARDI ANÁLISE DA MORFOLOGIA, VIABILIDADE
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCIELE OSMARINI LUNARDI ANÁLISE DA MORFOLOGIA, VIABILIDADE
CAMILA BIZARRO DA SILVA
 CENTRO DE PESQUISA EM CIÊNCIAS AGRÁRIAS MESTRADO EM SAÚDE E PRODUÇÃO DE RUMINANTES CAMILA BIZARRO DA SILVA EFEITO DE DIFERENTES CONCENTRAÇÕES DO HORMÔNIO FOLÍCULO ESTIMULANTE (FSH) NO SISTEMA DE CULTIVO
CENTRO DE PESQUISA EM CIÊNCIAS AGRÁRIAS MESTRADO EM SAÚDE E PRODUÇÃO DE RUMINANTES CAMILA BIZARRO DA SILVA EFEITO DE DIFERENTES CONCENTRAÇÕES DO HORMÔNIO FOLÍCULO ESTIMULANTE (FSH) NO SISTEMA DE CULTIVO
Gametogênese ESPERMATOGÊNESE. Prof a. Marta G. Amaral, Dra. Embriologia molecular
 Gametogênese ESPERMATOGÊNESE Prof a. Marta G. Amaral, Dra. Embriologia molecular Gametogênese Embrião final 3ªsemana CGP se formam no epiblasto na 2ª sem. Na 4ª sem. migram do o saco vitelino para as gônadas
Gametogênese ESPERMATOGÊNESE Prof a. Marta G. Amaral, Dra. Embriologia molecular Gametogênese Embrião final 3ªsemana CGP se formam no epiblasto na 2ª sem. Na 4ª sem. migram do o saco vitelino para as gônadas
Strategies for the management of the OHSS: results of freezing-all cycles
 1/28 Strategies for the management of the OHSS: results of freezing-all cycles Edson Borges Jr., Daniela Paes Almeida Ferreira Braga, Amanda S Setti, Livia Silva Vingris, Rita Cássia S Figueira, Assumpto
1/28 Strategies for the management of the OHSS: results of freezing-all cycles Edson Borges Jr., Daniela Paes Almeida Ferreira Braga, Amanda S Setti, Livia Silva Vingris, Rita Cássia S Figueira, Assumpto
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 i UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCISCO LÉO NASCIMENTO DE AGUIAR CULTIVO IN VITRO
i UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS FRANCISCO LÉO NASCIMENTO DE AGUIAR CULTIVO IN VITRO
benefícios oriundos de uma melhor compreensão do desenvolvimento folicular para a melhoria da eficiência reprodutiva dos caprinos também são
 1. INTRODUÇÃO Os caprinos são considerados animais economicamente atrativos por serem importantes fontes de carne, leite e pele. Além de estarem presentes em todos os continentes, no Brasil, eles desempenham
1. INTRODUÇÃO Os caprinos são considerados animais economicamente atrativos por serem importantes fontes de carne, leite e pele. Além de estarem presentes em todos os continentes, no Brasil, eles desempenham
Diferenças metabólicas e endócrinas entre femêas Bos taurus e Bos indicus e o impacto da interação da nutrição com a reprodução
 Diferenças metabólicas e endócrinas entre femêas Bos taurus e Bos indicus e o impacto da interação da nutrição com a reprodução Apresentadores: Med. Vet. Andressa Stein Maffi Kauana Soares FI: 1,79 Qual
Diferenças metabólicas e endócrinas entre femêas Bos taurus e Bos indicus e o impacto da interação da nutrição com a reprodução Apresentadores: Med. Vet. Andressa Stein Maffi Kauana Soares FI: 1,79 Qual
Núcleo celular: O centro de comando. Unidade 4 Pág 34
 Núcleo celular: O centro de comando. Unidade 4 Pág 34 NÚCLEO O núcleo é o centro de coordenação das atividades da célula. Em geral há um núcleo por célula; células sem núcleo são apenas uma fase da vida;
Núcleo celular: O centro de comando. Unidade 4 Pág 34 NÚCLEO O núcleo é o centro de coordenação das atividades da célula. Em geral há um núcleo por célula; células sem núcleo são apenas uma fase da vida;
Rede de Pesquisa do Ovário Artificial
 Rede de Pesquisa do Ovário Artificial Coordenador Instituição Executora Vigência Número de pesquisadores envolvidos Projetos associados a Rede/Coordenadores Importância da tecnologia do ovário artificial
Rede de Pesquisa do Ovário Artificial Coordenador Instituição Executora Vigência Número de pesquisadores envolvidos Projetos associados a Rede/Coordenadores Importância da tecnologia do ovário artificial
SANELY LOURENÇO DA COSTA CALIMAN
 SANELY LOURENÇO DA COSTA CALIMAN hfsh, T4 E IGF-I NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa
SANELY LOURENÇO DA COSTA CALIMAN hfsh, T4 E IGF-I NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa
ESTRATÉGIAS PARA A CRIOPRESERVAÇÃO DE CÉLULAS TRONCO MESENQUIMAIS
 1 ESTRATÉGIAS PARA A CRIOPRESERVAÇÃO DE CÉLULAS TRONCO MESENQUIMAIS CAMILA YAMAGUTI LENOCH 1, RAQUEL SILVA ALVES 2, IALANNA GARGHETTI SPILMANN 2, FERNANDA BOLDO SOUZA 2, LARISSA GOLFETTO DRUMM 2, UBIRAJARA
1 ESTRATÉGIAS PARA A CRIOPRESERVAÇÃO DE CÉLULAS TRONCO MESENQUIMAIS CAMILA YAMAGUTI LENOCH 1, RAQUEL SILVA ALVES 2, IALANNA GARGHETTI SPILMANN 2, FERNANDA BOLDO SOUZA 2, LARISSA GOLFETTO DRUMM 2, UBIRAJARA
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS SIMONE VIEIRA CASTRO CRIOPRESERVAÇÃO DE TECIDO OVARIANO
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS SIMONE VIEIRA CASTRO CRIOPRESERVAÇÃO DE TECIDO OVARIANO
Interação dos mecanismos fisiológicos que influenciam a fertilidade em vacas leiteiras
 Ministério da Educação Universidade Federal de Pelotas Faculdade de Medicina Veterinária Núcleo de Ensino, Pesquisa e Extensão em Pecuária-NUPEEC Interação dos mecanismos fisiológicos que influenciam a
Ministério da Educação Universidade Federal de Pelotas Faculdade de Medicina Veterinária Núcleo de Ensino, Pesquisa e Extensão em Pecuária-NUPEEC Interação dos mecanismos fisiológicos que influenciam a
28/04/2010. Espermatozóides são formados e lançados no espaço do tubos. Tubos Seminíferos. Epidídimo. Testículo Células em divisão (mitose x meiose)
 GAMETOGÊNESE - -OVOGÊNESE gustavosimas@gmail.com Tubos Seminíferos Espermatozóides são formados e lançados no espaço do tubos Epidídimo Testículo Células em divisão (mitose x meiose) 1 EPIDÍDIMO (armazena
GAMETOGÊNESE - -OVOGÊNESE gustavosimas@gmail.com Tubos Seminíferos Espermatozóides são formados e lançados no espaço do tubos Epidídimo Testículo Células em divisão (mitose x meiose) 1 EPIDÍDIMO (armazena
A g r u p a m e n t o d e E s c o l a s A n t ó n i o S é r g i o V. N. G a i a E S C O L A S E C U N D Á R I A / 3 A N T Ó N I O S É R G I O
 A g r u p a m e n t o d e E s c o l a s A n t ó n i o S é r g i o V. N. G a i a E S C O L A S E C U N D Á R I A / 3 A N T Ó N I O S É R G I O BIOLOGIA Módulo 1 12º CTec CURSO CIENTÍFICO-HUMANÍSTICO DE
A g r u p a m e n t o d e E s c o l a s A n t ó n i o S é r g i o V. N. G a i a E S C O L A S E C U N D Á R I A / 3 A N T Ó N I O S É R G I O BIOLOGIA Módulo 1 12º CTec CURSO CIENTÍFICO-HUMANÍSTICO DE
Pontifícia Universidade Católica de Goiás Departamento de Biologia. Ciclo celular. Prof. Msc. Macks Wendhell Gonçalves
 Pontifícia Universidade Católica de Goiás Departamento de Biologia Ciclo celular Prof. Msc. Macks Wendhell Gonçalves Mitose e Meiose Conceitos: - Mitose e meiose é responsável pela continuidade genética
Pontifícia Universidade Católica de Goiás Departamento de Biologia Ciclo celular Prof. Msc. Macks Wendhell Gonçalves Mitose e Meiose Conceitos: - Mitose e meiose é responsável pela continuidade genética
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ROBERTA NOGUEIRA CHAVES EFEITO DA TEMPERATURA E DO
1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS ROBERTA NOGUEIRA CHAVES EFEITO DA TEMPERATURA E DO
GILDAS MBEMYA TETAPING
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS GILDAS MBEMYA TETAPING EXTRATO AQUOSO DE JUSTICIA INSULARIS:
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS GILDAS MBEMYA TETAPING EXTRATO AQUOSO DE JUSTICIA INSULARIS:
PROGRESSÃO MEIÓTICA NA MATURAÇÃO NUCLEAR IN VITRO DE OÓCITOS BOVINOS DE OVÁRIOS COM E SEM A PRESENÇA DE CORPO LÚTEO
 PROGRESSÃO MEIÓTICA NA MATURAÇÃO NUCLEAR IN VITRO DE OÓCITOS BOVINOS DE OVÁRIOS COM E SEM A PRESENÇA DE CORPO LÚTEO Janaina C. Medeiros FONSECA* 1, Leandro Polato LAZARI¹, Matheus montanari de SOUZA 1,
PROGRESSÃO MEIÓTICA NA MATURAÇÃO NUCLEAR IN VITRO DE OÓCITOS BOVINOS DE OVÁRIOS COM E SEM A PRESENÇA DE CORPO LÚTEO Janaina C. Medeiros FONSECA* 1, Leandro Polato LAZARI¹, Matheus montanari de SOUZA 1,
A Célula Humana. Disciplina: Anatomia e Fisiologia. Samara Cristina Ferreira Machado. Programa Nacional de Formação em Radioterapia
 Disciplina: Anatomia e Fisiologia A Célula Humana Samara Cristina Ferreira Machado Programa Nacional de Formação em Radioterapia Abordagem Celular - Estrutura Celular - Função Celular - Ciclo Celular Estrutura
Disciplina: Anatomia e Fisiologia A Célula Humana Samara Cristina Ferreira Machado Programa Nacional de Formação em Radioterapia Abordagem Celular - Estrutura Celular - Função Celular - Ciclo Celular Estrutura
RAPHAEL FERNANDO BRAGA GONÇALVES
 RAPHAEL FERNANDO BRAGA GONÇALVES AVALIAÇÃO DA MORFOLOGIA E VIABILIDADE DE FOLÍCULOS PRÉ- ANTRAIS OVINOS APÓS CRIOPRESERVAÇÃO E CULTIVO IN VITRO DE CURTA DURAÇÃO FORTALEZA 2011 UNIVERSIDADE ESTADUAL DO
RAPHAEL FERNANDO BRAGA GONÇALVES AVALIAÇÃO DA MORFOLOGIA E VIABILIDADE DE FOLÍCULOS PRÉ- ANTRAIS OVINOS APÓS CRIOPRESERVAÇÃO E CULTIVO IN VITRO DE CURTA DURAÇÃO FORTALEZA 2011 UNIVERSIDADE ESTADUAL DO
Viviane Rohrig Rabassa Prof a. Assist., Doutoranda em Veterinária, UFPel. Marcio Nunes Corrêa Prof. Adjunto, FV-UFPel
 Universidade Federal de Pelotas Programa de Pós-graduação em Veterinária Núcleo de Pesquisa, Ensino e Extensão em Pecuária Viviane Rohrig Rabassa Prof a. Assist., Doutoranda em Veterinária, UFPel Marcio
Universidade Federal de Pelotas Programa de Pós-graduação em Veterinária Núcleo de Pesquisa, Ensino e Extensão em Pecuária Viviane Rohrig Rabassa Prof a. Assist., Doutoranda em Veterinária, UFPel Marcio
INFLUÊNCIA DA ORIGEM E PUREZA DO HORMÔNIO FOLÍCULO ESTIMULANTE (FSH) NA SOBREVIVÊNCIA E NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS
 INFLUÊNCIA DA ORIGEM E PUREZA DO HORMÔNIO FOLÍCULO ESTIMULANTE (FSH) NA SOBREVIVÊNCIA E NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS 1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO
INFLUÊNCIA DA ORIGEM E PUREZA DO HORMÔNIO FOLÍCULO ESTIMULANTE (FSH) NA SOBREVIVÊNCIA E NO DESENVOLVIMENTO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS CAPRINOS 1 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO
ASSOCIAÇÃO DAS TÉCNICAS DE CRIOPRESERVAÇÃO E CULTIVO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS PARA A OBTENÇÃO DE EMBRIÕES CAPRINOS.
 ASSOCIAÇÃO DAS TÉCNICAS DE CRIOPRESERVAÇÃO E CULTIVO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS PARA A OBTENÇÃO DE EMBRIÕES CAPRINOS. (Cryopreservation and in vitro culture of ovarian preantral follicles to obtaining
ASSOCIAÇÃO DAS TÉCNICAS DE CRIOPRESERVAÇÃO E CULTIVO IN VITRO DE FOLÍCULOS PRÉ-ANTRAIS PARA A OBTENÇÃO DE EMBRIÕES CAPRINOS. (Cryopreservation and in vitro culture of ovarian preantral follicles to obtaining
REPRODUÇÃO HUMANA. Lásaro Henrique
 REPRODUÇÃO HUMANA Lásaro Henrique GAMETOGÊNESE Processo de formação de gametas. Pode ser: Espermatogênese Ovulogênese ESPERMATOGÊNESE Ocorre nos tubos seminíferos,das paredes para a luz de cada tubo. ETAPAS
REPRODUÇÃO HUMANA Lásaro Henrique GAMETOGÊNESE Processo de formação de gametas. Pode ser: Espermatogênese Ovulogênese ESPERMATOGÊNESE Ocorre nos tubos seminíferos,das paredes para a luz de cada tubo. ETAPAS
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS GIOVANNA QUINTINO RODRIGUES EFEITO DE DIFERENTES CONCENTRAÇÕES
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS GIOVANNA QUINTINO RODRIGUES EFEITO DE DIFERENTES CONCENTRAÇÕES
BIOTÉCNICAS REPRODUTIVAS E BEM-ESTAR ANIMAL
 70 BIOTÉCNICAS REPRODUTIVAS E BEM-ESTAR ANIMAL José Ricardo FIGUEIREDO 1 e Lúcia Daniel Machado da SILVA 2 Resumo - Considera-se inegável a contribuição que as biotécnicas reprodutivas têm dado ao desenvolvimento
70 BIOTÉCNICAS REPRODUTIVAS E BEM-ESTAR ANIMAL José Ricardo FIGUEIREDO 1 e Lúcia Daniel Machado da SILVA 2 Resumo - Considera-se inegável a contribuição que as biotécnicas reprodutivas têm dado ao desenvolvimento
Avaliação do Índice Apoptótico em Adenomas Pleomórficos de Glândulas Salivares
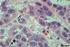 PONTIFÍCIA UNIVERSIDADE CATÓLICA DE MINAS GERAIS Faculdade de Odontologia Avaliação do Índice Apoptótico em Adenomas Pleomórficos de Glândulas Salivares Paulo César de Lacerda Dantas Belo Horizonte- MG
PONTIFÍCIA UNIVERSIDADE CATÓLICA DE MINAS GERAIS Faculdade de Odontologia Avaliação do Índice Apoptótico em Adenomas Pleomórficos de Glândulas Salivares Paulo César de Lacerda Dantas Belo Horizonte- MG
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS JESÚS DE LOS REYES CADENAS MORENO CULTIVO IN VITRO DE
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS JESÚS DE LOS REYES CADENAS MORENO CULTIVO IN VITRO DE
Fertilização. Prof a. Marta G. Amaral, Dra. Embriologia molecular
 Fertilização Prof a. Marta G. Amaral, Dra. Embriologia molecular Geralmente ocorre na ampola da trompa uterina. Não ocorre no útero. Sêmen Formado pelos espermatozoides e mistura de secreções testiculares,
Fertilização Prof a. Marta G. Amaral, Dra. Embriologia molecular Geralmente ocorre na ampola da trompa uterina. Não ocorre no útero. Sêmen Formado pelos espermatozoides e mistura de secreções testiculares,
BLOQUEIO DE OÓCITOS DE BORREGAS EM VESÍCULA GERMINATIVA COM ROSCOVITINA
 BLOQUEIO DE OÓCITOS DE BORREGAS EM VESÍCULA GERMINATIVA COM ROSCOVITINA Raphael Colombo GASPAR* 1, Sony Dimas BICUDO 2, Fernanda da Cruz LANDIM- ALVARENGA 2 ; Letícia Ferrari CROCOMO 1, Sergio LEDDA 3.
BLOQUEIO DE OÓCITOS DE BORREGAS EM VESÍCULA GERMINATIVA COM ROSCOVITINA Raphael Colombo GASPAR* 1, Sony Dimas BICUDO 2, Fernanda da Cruz LANDIM- ALVARENGA 2 ; Letícia Ferrari CROCOMO 1, Sergio LEDDA 3.
GÔNADAS = glândulas endócrinas com funções de sustentar o desenvolvimento e a maturação das células germinativas e sintetizar e secretar hormônios
 Fisiologia do Sistema Reprodutor Fisiologia do Sistema Reprodutor GÔNADAS = glândulas endócrinas com funções de sustentar o desenvolvimento e a maturação das células germinativas e sintetizar e secretar
Fisiologia do Sistema Reprodutor Fisiologia do Sistema Reprodutor GÔNADAS = glândulas endócrinas com funções de sustentar o desenvolvimento e a maturação das células germinativas e sintetizar e secretar
Sistema Reprodutor Feminino. Acadêmica de Veterinária Carolina Wickboldt Fonseca
 Sistema Reprodutor Feminino Acadêmica de Veterinária Carolina Wickboldt Fonseca Introdução Apresento este trabalho sobre o Sistema Reprodutor Feminino (SRF), histologicamente, descrevendo algumas estruturas
Sistema Reprodutor Feminino Acadêmica de Veterinária Carolina Wickboldt Fonseca Introdução Apresento este trabalho sobre o Sistema Reprodutor Feminino (SRF), histologicamente, descrevendo algumas estruturas
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS JOHANNA LEIVA REVILLA Teste de toxicidade da fração da
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS JOHANNA LEIVA REVILLA Teste de toxicidade da fração da
ENDOCRINOLOGIA DA REPRODUÇÃO. Elisângela Mirapalheta Madeira Medica Veterinária, MC
 ENDOCRINOLOGIA DA REPRODUÇÃO Elisângela Mirapalheta Madeira Medica Veterinária, MC Introdução Glândulas Endócrinas Hipotálamo Hipófise Gônadas Glândula pineal Glândulas Endócrinas Hipotálamo Glândulas
ENDOCRINOLOGIA DA REPRODUÇÃO Elisângela Mirapalheta Madeira Medica Veterinária, MC Introdução Glândulas Endócrinas Hipotálamo Hipófise Gônadas Glândula pineal Glândulas Endócrinas Hipotálamo Glândulas
ANÁLISE MORFOLÓGICA E ULTRAESTRUTURAL DE FOLÍCULOS PRIMORDIAIS OVINOS CONSERVADOS EM SOLUÇÃO SALINA 0,9% E TCM
 Universidade Estadual do Ceará Pró-Reitoria de Pós-Graduação e Pesquisa Faculdade de Veterinária Programa de Pós-Graduação em Ciências Veterinárias Maria Helena Tavares de Matos ANÁLISE MORFOLÓGICA E ULTRAESTRUTURAL
Universidade Estadual do Ceará Pró-Reitoria de Pós-Graduação e Pesquisa Faculdade de Veterinária Programa de Pós-Graduação em Ciências Veterinárias Maria Helena Tavares de Matos ANÁLISE MORFOLÓGICA E ULTRAESTRUTURAL
26 a 29 de novembro de 2013 Campus de Palmas
 AVALIAÇÃO DE ESTRUTURAS EMBRIONÁRIAS DE BOVINOS PÓS VITRIFICAÇÃO NA REGIÃO NORTE DO TOCANTINS Karina Almeida Maciel 1 ; Márcio Gianordoli Teixeira Gomes 2 ; Francisca Elda Ferreira Dias 3, 1 Aluno do Curso
AVALIAÇÃO DE ESTRUTURAS EMBRIONÁRIAS DE BOVINOS PÓS VITRIFICAÇÃO NA REGIÃO NORTE DO TOCANTINS Karina Almeida Maciel 1 ; Márcio Gianordoli Teixeira Gomes 2 ; Francisca Elda Ferreira Dias 3, 1 Aluno do Curso
HORMÔNIOS VEGETAIS. Katia Christina Zuffellato-Ribas
 HORMÔNIOS VEGETAIS Katia Christina Zuffellato-Ribas HORMÔNIO VEGETAL COMPOSTO ORGÂNICO, NÃO NUTRIENTE, DE OCORRÊNCIA NATURAL, PRODUZIDO NA PLANTA, O QUAL, EM BAIXAS CONCENTRAÇÕES (10-4 A 10-6 M), PROMOVE,
HORMÔNIOS VEGETAIS Katia Christina Zuffellato-Ribas HORMÔNIO VEGETAL COMPOSTO ORGÂNICO, NÃO NUTRIENTE, DE OCORRÊNCIA NATURAL, PRODUZIDO NA PLANTA, O QUAL, EM BAIXAS CONCENTRAÇÕES (10-4 A 10-6 M), PROMOVE,
CICLO MENSTRUAL CICLO OVARIANO CICLO UTERINO. Prof.ª Patrícia Silva de Medeiros Ajes - Guarantã do Norte/MT
 CICLO MENSTRUAL CICLO OVARIANO CICLO UTERINO Prof.ª Patrícia Silva de Medeiros Ajes - Guarantã do Norte/MT Ciclo Menstrual Fisiologia do Ciclo Menstrual A primeira menstruação da vida da mulher chama-
CICLO MENSTRUAL CICLO OVARIANO CICLO UTERINO Prof.ª Patrícia Silva de Medeiros Ajes - Guarantã do Norte/MT Ciclo Menstrual Fisiologia do Ciclo Menstrual A primeira menstruação da vida da mulher chama-
Ciclo Reprodutivo Feminino Parte I. Embriogênese do aparelho reprodutor feminino e desenvolvimento puberal
 Ciclo Reprodutivo Feminino Parte I. Embriogênese do aparelho reprodutor feminino e desenvolvimento puberal Disciplina de Reprodução Humana Departamento de Ginecologia e Obstetrícia FMRP - USP Profa Dra
Ciclo Reprodutivo Feminino Parte I. Embriogênese do aparelho reprodutor feminino e desenvolvimento puberal Disciplina de Reprodução Humana Departamento de Ginecologia e Obstetrícia FMRP - USP Profa Dra
CROMOSSOMOS. Profa. Dra. Viviane Nogaroto
 CROMOSSOMOS Cromossomos EUCARIOTOS Cada cromossomo carrega um subconjunto de genes que são arranjados linearmente ao longo do DNA Tamanho: -DNA de cromossomo de E. coli: 1.200 µm -DNA de cromossomo humano:
CROMOSSOMOS Cromossomos EUCARIOTOS Cada cromossomo carrega um subconjunto de genes que são arranjados linearmente ao longo do DNA Tamanho: -DNA de cromossomo de E. coli: 1.200 µm -DNA de cromossomo humano:
Ciclo Celular. Prof. Tiago Collares, Dr. (MSN) ( )
 Ciclo Celular Prof. Tiago Collares, Dr. tiago_collares@hotmail.com (MSN) collares.t@gmail.com (e-mail ) O Ciclo Celular Toda célula se origina da divisão de uma célula preexistente; Nos eucariontes, o
Ciclo Celular Prof. Tiago Collares, Dr. tiago_collares@hotmail.com (MSN) collares.t@gmail.com (e-mail ) O Ciclo Celular Toda célula se origina da divisão de uma célula preexistente; Nos eucariontes, o
Avanços, aplicações, limitações e perspectivas da tecnologia do ovário artificial de ruminantes
 Anais do IX Congresso Norte e Nordeste de Reprodução Animal (CONERA 2018); Belém, PA, 10 a 12 de setembro de 2018. Avanços, aplicações, limitações e perspectivas da tecnologia do ovário artificial de ruminantes
Anais do IX Congresso Norte e Nordeste de Reprodução Animal (CONERA 2018); Belém, PA, 10 a 12 de setembro de 2018. Avanços, aplicações, limitações e perspectivas da tecnologia do ovário artificial de ruminantes
Os cartões à seguir devem ser recortados, dobrados ao meio e colados.
 Os cartões à seguir devem ser recortados, dobrados ao meio e colados. CASO eles se formem? Como as células das membranas entre os dedos de um feto desaparecem para que Embrião com cerca de 42 dias: apresenta
Os cartões à seguir devem ser recortados, dobrados ao meio e colados. CASO eles se formem? Como as células das membranas entre os dedos de um feto desaparecem para que Embrião com cerca de 42 dias: apresenta
Gametogênese e fecundação
 Gametogênese e fecundação Objetivos os estudantes deverão ser capazes de descrever as etapas da meiose (meiose I e meiose II) e explicar a função do processo meiótico descrever o processo de formação dos
Gametogênese e fecundação Objetivos os estudantes deverão ser capazes de descrever as etapas da meiose (meiose I e meiose II) e explicar a função do processo meiótico descrever o processo de formação dos
DIVISÃO CELULAR. Multiplicação celular:
 DIVISÃO CELULAR Multiplicação celular: Para que uma célula se multiplique o genoma também precisa se multiplicar REPLICAÇÃO do DNA precede multiplicação celular DIVISÃO CELULAR MITOSE: divisão das células
DIVISÃO CELULAR Multiplicação celular: Para que uma célula se multiplique o genoma também precisa se multiplicar REPLICAÇÃO do DNA precede multiplicação celular DIVISÃO CELULAR MITOSE: divisão das células
ESCOLA ESTADUAL DR JOSÉ MARQUES DE OLIVEIRA AVALIAÇÃO ESTUDOS INDEPENDENTES DE RECUPERAÇÃO RESOLUÇÃO SEE Nº 2.197, DE 26 DE OUTUBRO DE 2012
 ESCOLA ESTADUAL DR JOSÉ MARQUES DE OLIVEIRA AVALIAÇÃO ESTUDOS INDEPENDENTES DE RECUPERAÇÃO RESOLUÇÃO SEE Nº 2.197, DE 26 DE OUTUBRO DE 2012 Aluno: Ano:2016 Série: 1ºAno Data : Matéria: Biologia Turno:
ESCOLA ESTADUAL DR JOSÉ MARQUES DE OLIVEIRA AVALIAÇÃO ESTUDOS INDEPENDENTES DE RECUPERAÇÃO RESOLUÇÃO SEE Nº 2.197, DE 26 DE OUTUBRO DE 2012 Aluno: Ano:2016 Série: 1ºAno Data : Matéria: Biologia Turno:
UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS
 0 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS LUCIANA ROCHA FAUSTINO DIFERENTES CONCENTRAÇÕES E TEMPOS
0 UNIVERSIDADE ESTADUAL DO CEARÁ PRÓ-REITORIA DE PÓS-GRADUAÇÃO E PESQUISA FACULDADE DE VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS VETERINÁRIAS LUCIANA ROCHA FAUSTINO DIFERENTES CONCENTRAÇÕES E TEMPOS
Painel temático. Pelotas, 28 de setembro de 2017
 Painel temático Pelotas, 28 de setembro de 2017 Universidade Federal de Pelotas Faculdade De Veterinária Núcleo de Pesquisa, Ensino e Extensão em Pecuária www.ufpel.edu.br/nupeec Painel temático Efeitos
Painel temático Pelotas, 28 de setembro de 2017 Universidade Federal de Pelotas Faculdade De Veterinária Núcleo de Pesquisa, Ensino e Extensão em Pecuária www.ufpel.edu.br/nupeec Painel temático Efeitos
